Chemistry > GIZMOS > Gizmos _ Student Exploration_Calorimetry Lab_2020 | M11L2M1 Calorimetry Lab (All)
Gizmos _ Student Exploration_Calorimetry Lab_2020 | M11L2M1 Calorimetry Lab
Document Content and Description Below
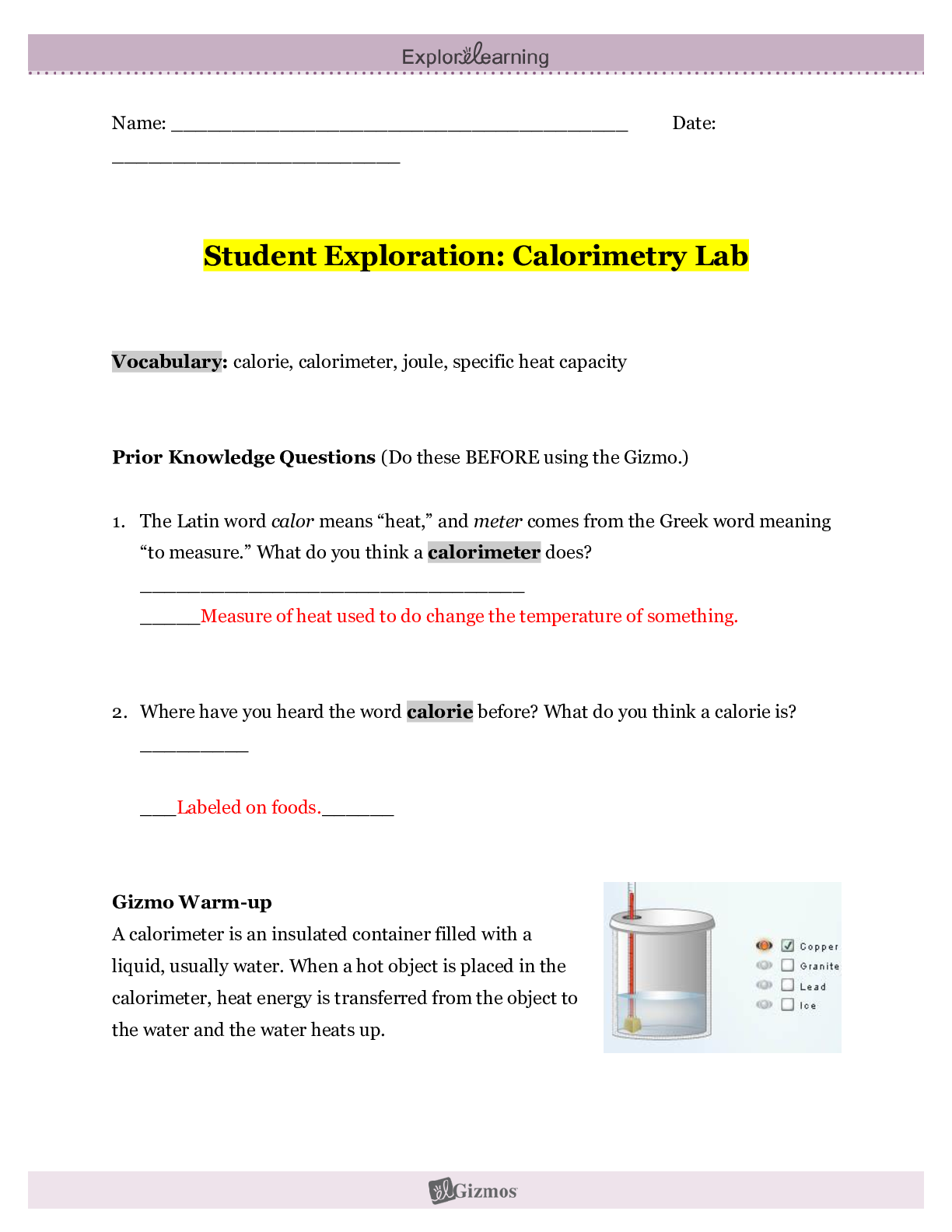
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “he... at,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter does? Measures heat. 2. Where have you heard the word calorie before? What do you think a calorie is? With food, the amount of energy it takes to heat up one ml of water. Gizmo Warm-up A calorimeter is an insulated container filled with a liquid, usually water. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat capacity. You will use the Calorimetry Lab Gizmo™ to determine the specific heat capacities of various substances. 1. On the SIMULATION pane, select Copper. Use the slider to set its Mass t o 200 g. Set the Water mass to 200 g. Check that the Water temp is set to 30.0 °C and the copper’s Temp is 90 °C. Select the GRAPH tab, and click Play ( ). A. What was the Final temperature of the copper and the water? 34.96C B. How much did the temperature of the copper change? 55.04C C. How much did the temperature of the water change? 4.96C 2. Specific heat capacity can be described as a substance’s resistance to temperature changes. Which substance has a greater specific heat capacity, copper or water? Explain. Water, the temperature of the water changed the least amount, therefore its resistance is greater. Activity A: Heat transfer Get the Gizmo ready: ● Click Reset ( ). Question: What factors determine how heat energy transfers between objects? 1. Predict: In the Gizmo warm-up, you saw how 200 g of 90 °C copper transfers heat to 200 g of 30.0 °C water. A. How do you think increasing the water’s mass would affect the final temperature? It would decrease the final temperature B. How do you think decreasing the copper’s mass would affect the final temperature? It would also decrease the final temperature. C. How do you think increasing or decreasing the copper’s initial temperature would affect the final temperature? Increasing it would increase the final temperature, and decreasing it would decrease the final temperature. 2. Collect data: Use the Gizmo to determine the final temperature for each set-up listed below. Record your results in the tables. In the first table, you experiment with changing the water’s mass. In the second table, you change the copper’s mass. In the third table, you change the initial temperature of the copper. The first row of each table has been completed for you. Copper Water Final Temp. (°C) Initial Temp. (°C) Mass (g) Initial Temp. (°C) Mass (g) 3. 90 °C 200 g 30.0 °C 200 g 34.96 °C 90 °C 200 g 30.0 °C 2,000 g 30.54C 4. 90 °C 200 g 30.0 °C 200 g 34.96 °C 90 °C 20 g 30.0 °C 200 g 30.54C 5. 90 °C 200 g 30.0 °C 200 g 34.96 °C 100 °C 200 g 30.0 °C 200 g 35.79C 50 °C 200 g 30.0 °C 200 g 31.65C (Activity A continued on next page) Activity A (continued from previous page) 3. Analyze: For each factor listed in the chart below, explain how the final temperature was changed and why you think that change occurred. A. What was the effect of increasing the water’s mass? It decreased the final temperature, because there was more to warm up. B. What was the effect of decreasing the copper’s mass? It decreased the final temperature, the copper was warming up the water past 30C and when you take more of that warmth out, the water won’t heat up as much. C. What was the effect of changing the initial temperature of the copper? The higher the initial temperature of copper, the higher the final temperature. 4. Draw conclusions: The amount that the water’s temperature increases depends on the mass of the water and the amount o [Show More]
Last updated: 1 year ago
Preview 1 out of 12 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 10, 2022
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Apr 10, 2022
Downloads
0
Views
77