Gizmos _ Kami_Export_Copy_of_MolesSE _2020 | Kami_Export_Copy_of_MolesSE
Document Content and Description Below
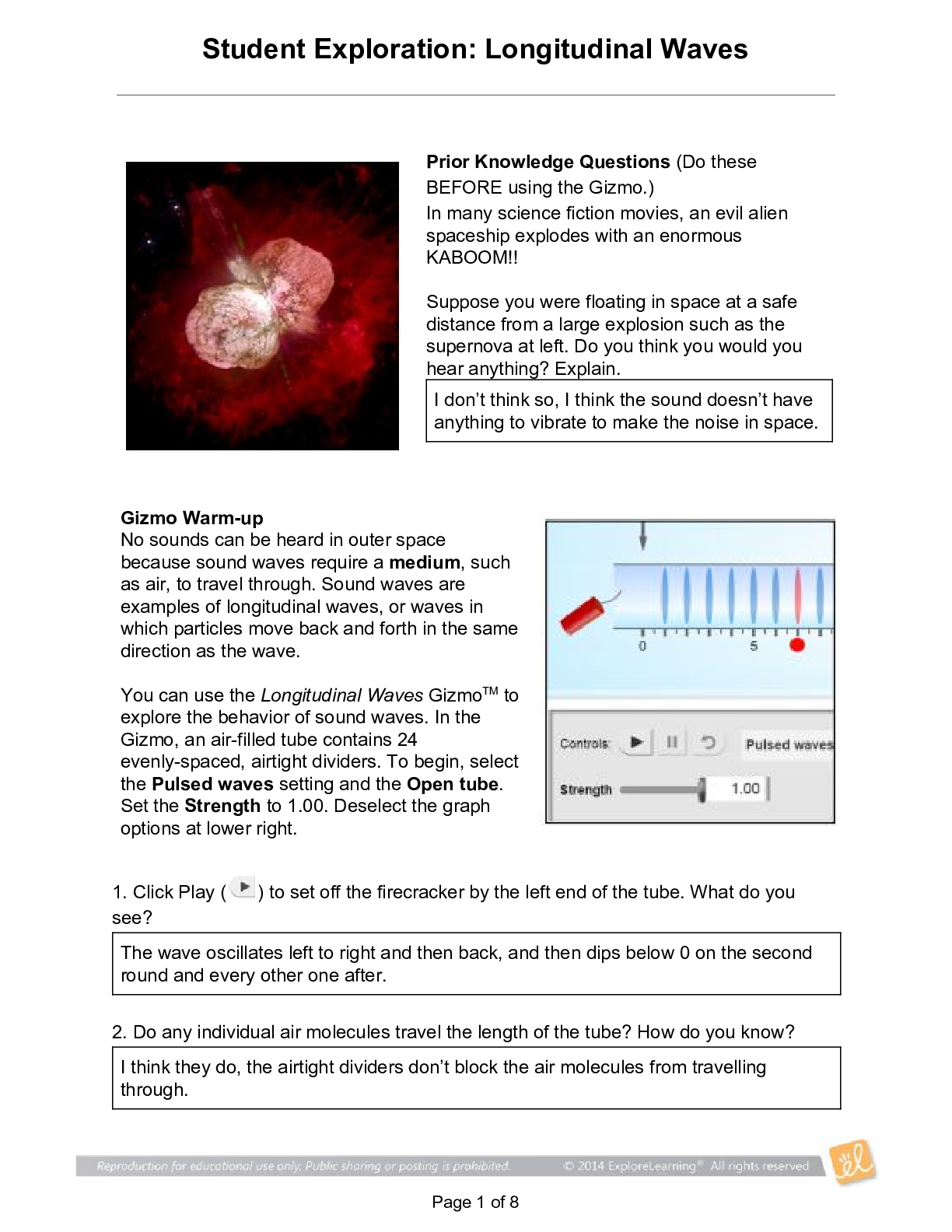
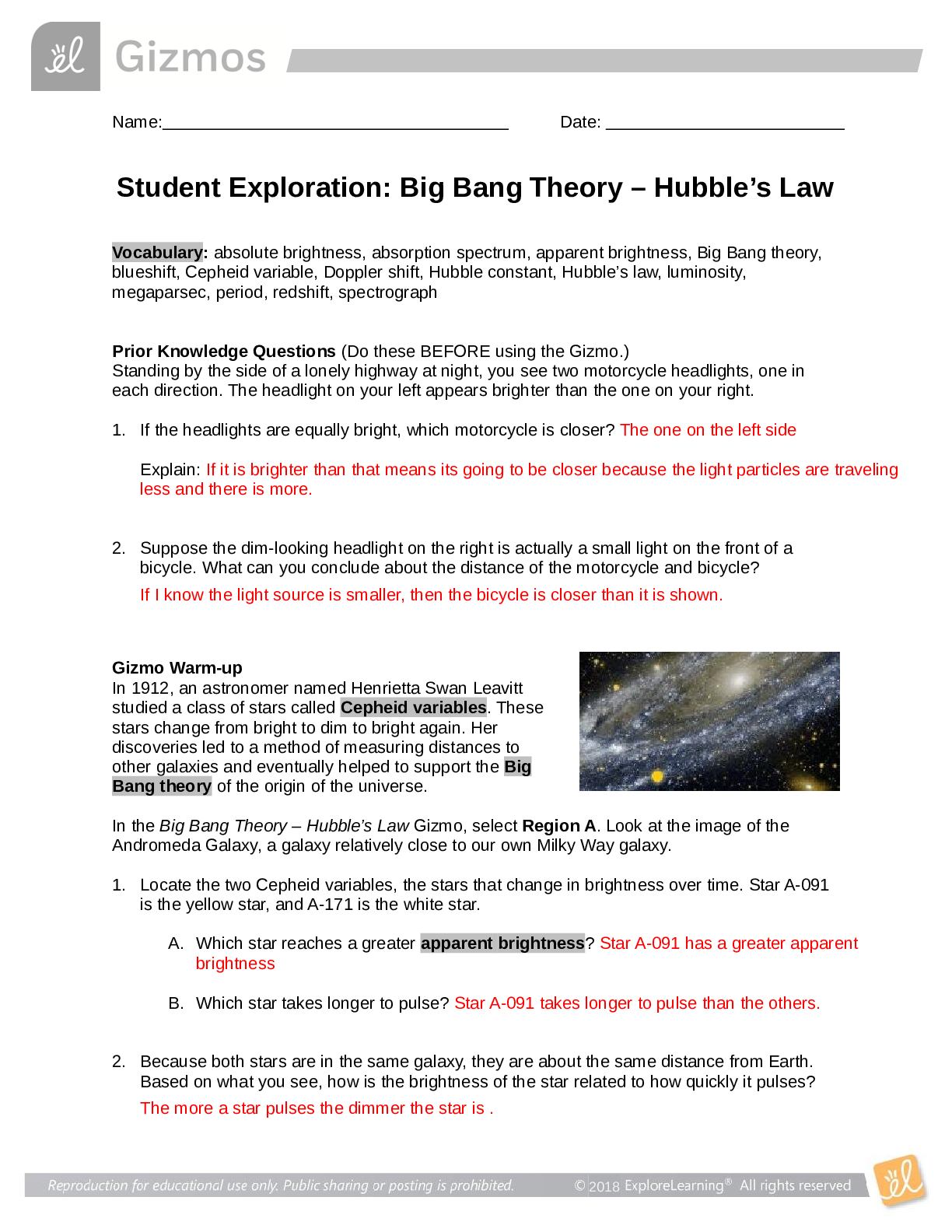
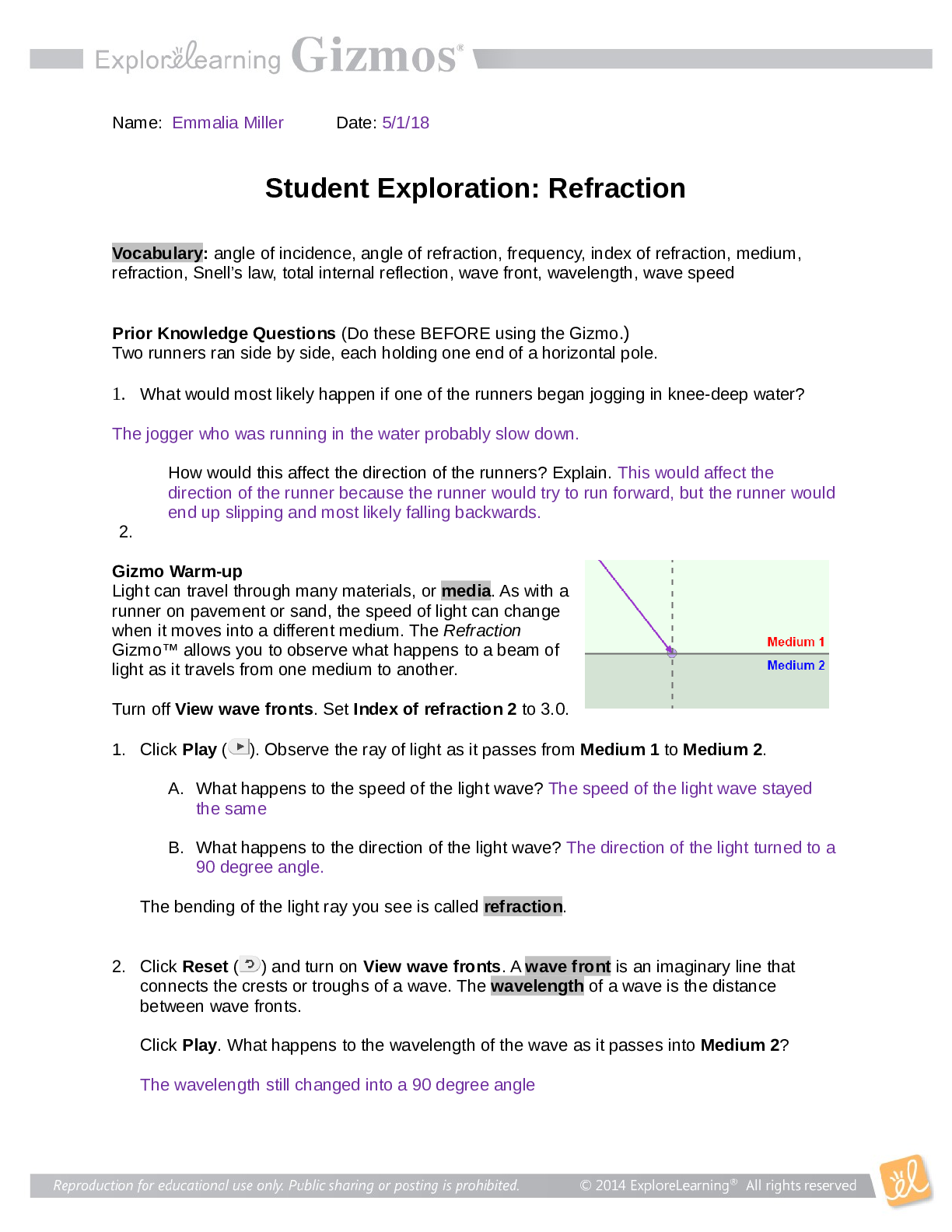
Name: Ashley Ceredon Date: Sep 24 2020 Student Exploration: Moles Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabu... lary: atomic mass, Avogadro constant, conversion factor, dimensional analysis, mole, molar mass, molecular mass, scientific notation, significant figures, unified atomic mass unit Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. In the image to the right, note a dozen eggs, a dozen donuts and a dozen roses. How many of each item do you have? 12 2. Would a dozen of each object have the same mass? They would have different masses because they are all different objects. 3. Suppose you have a dozen carbon atoms, a dozen gold atoms, and a dozen iron atoms. Even though you have the same number of each, would you expect them all to have the same mass? Explain. I would not expect all atoms to have the same mass because atoms can be made from several different isotopes which all weigh different amounts. Gizmo Warm-up When counting roses, eggs, or donuts, a dozen is a good unit to use. If you are counting atoms, however, a dozen is not much help. In the Moles Gizmo, you will learn about a unit used to count atoms. On the AVOGADRO CONSTANT tab, place the copper (Cu) atom on the nano-balance on the left, which will show the average atomic mass of copper rather than the mass of a single copper atom. 1. What is the average mass of a copper atom? 63.546u The unit “u” refers to unified atomic mass units. A single proton or neutron has a mass of approximately one atomic mass unit. (Officially, 1 u is one-twelfth the mass of a C-12 atom.) 2. To gain an idea as to how many atoms are in a gram or so of copper, use the larger balance on the right. Press Add atoms to put a scoop of atoms in the weighing dish, and keep adding until the balance registers between 1 and 2 grams. If you don’t seem to be making much progress, adjust the exponent using the slider, which will make the scoop size bigger. How many atoms did you need to add? Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved 1 x 10^22 atoms to make 1.055g of Cu Activity A: Molar Mass Get the Gizmo ready: ● Select the AVOGADRO CONSTANT tab. ● Turn on Show hints and check that Copper (Cu) is selected. Introduction: Since atoms are so tiny, chemists have devised a unit known as the mole. A mole represents a macroscopic quantity of matter that can be used in the laboratory. One mole of any element has the same mass in grams as its atomic mass in u. Question: How many particles are in a mole? 1. Explore: Note the average atomic mass of copper on the nano-balance. Add atoms to the larger balance until it registers the same number (in g) as the reading on the nano-balance (in u). Use the Exponent slider to help get the correct amount. Stop adding atoms when the readings on both balances match exactly (to the nearest 0.001 g). How many atoms did you need to add? 1 x 10^19 2. Explore: Repeat the same procedure with carbon, then sulfur and aluminum. A. For each element, how many atoms did you need to add? Carbon : 3 x 10^19 Sulfur : 1 x 10^19 B. What do you notice about the number of atoms in one mole? 3. Discover: In each case, you measured out one mole of atoms, since the mass of one mole of any element, in grams, is equal to its atomic mass, in u. One mole of any element contains the same number of atoms, a number known as the Avogadro constant. What is the exact value of the Avogadro constant? 6.02 x 10^23 4. Illustrate: The Avogadro constant is so large it is normally written in scientific notation. To get an idea of the enormity of the Avogadro constant, write it out in standard form. (You will need to move the decimal place to the right 23 times, so you will need to add a lot of zeros!) 602214076000000000000000 Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved 5. Compare: While the number of atoms in a mole is constant, the number of grams in a mole changes based on the element. The number of grams in a mole (g/mol) is known as its molar mass, and has the same numerical value as an element’s atomic mass (in u). Use the Gizmo to find the atomic and molar mass of the following elements. Use proper units. S: Atomic mass 32.065u Molar mass 32.065g Al: Atomic mass 26.982u Molar mass 26.982g 6. Experiment: Select Copper(I) oxide, Cu2O. Note that Cu2O is a compound composed of different types of atoms bonded together. Place the Cu2O molecule on the nano-balance. A. What is the molecular mass of Cu2O? 143.091g B. Add molecules to the larger balance until its reading matches that of the nano-balance exactly. How many molecules did you need to add? 6.0221 x 10^23 C. Repeat the above procedure with another molecule of your choice. How many molecules did you need to add? 6.0220 x 10^23 7. Summarize: Complete the following statements: A. 1 mole of any element contains 6.0221 × 10 23 atoms B. 1 mole of any compound contains 6.0221 × 10 23 molecules 8. Extend: For compounds, it is sometimes necessary to calculate the number of atoms of each element within a molecule. Select Iron(II) chloride. Note the image of the molecule. A. How many Fe atoms are within a single FeCl2 molecule? 1 B. How many Cl atoms are within a single FeCl2 molecule? 2 C. Use the nano-balance to find the mass of each of these atoms: Mass of Fe atom: 55.845g Mass of Cl atom: 35.453g D. Find the sum of their masses (1 Fe atom + 2 Cl atoms): 126.751g Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved E. Place the FeCl2 molecule on the balance. Is the sum of the masses of the individual atoms equal to the molecular mass of the compound? No, because it only counts the mass for 1 chlorine atom when there is 2 chlorine atoms 9. Calculate: Select Copper(I) oxide. Note the image of the molecule. Place it on the balance. A. How many moles of copper would be needed to make 1 mole of Cu2O? 2 B. How many grams of copper would you need? 127.092g Grams of oxygen? 15.999g C. In addition to showing the ratio of atoms in a molecule, what else do the subscripts in a formula tell us? The subscripts tell us how many moles of each element in the compound is present. Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved Activity B: Conversions Get the Gizmo ready: ● Select the CONVERSIONS tab. ● Select Carbon (C). Introduction: Chemical formulas represent ratios. To make H2O, you need two atoms of H for each atom of O; you would also need two moles of H for every mole of O. However, when performing experiments in the lab, substances are measured in grams, not atoms or moles. Therefore, it is important to be able to convert freely between atoms, moles, and grams. Question: How do you convert particles to grams, a [Show More]
Last updated: 1 year ago
Preview 1 out of 8 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 11, 2022
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
Apr 11, 2022
Downloads
0
Views
97






.png)











.png)

