Chemistry > GIZMOS > Gizmos _ Student Exploration_Calorimetry Lab_2020 | M11L2M1 Calorimetry Lab (All)
Gizmos _ Student Exploration_Calorimetry Lab_2020 | M11L2M1 Calorimetry Lab
Document Content and Description Below
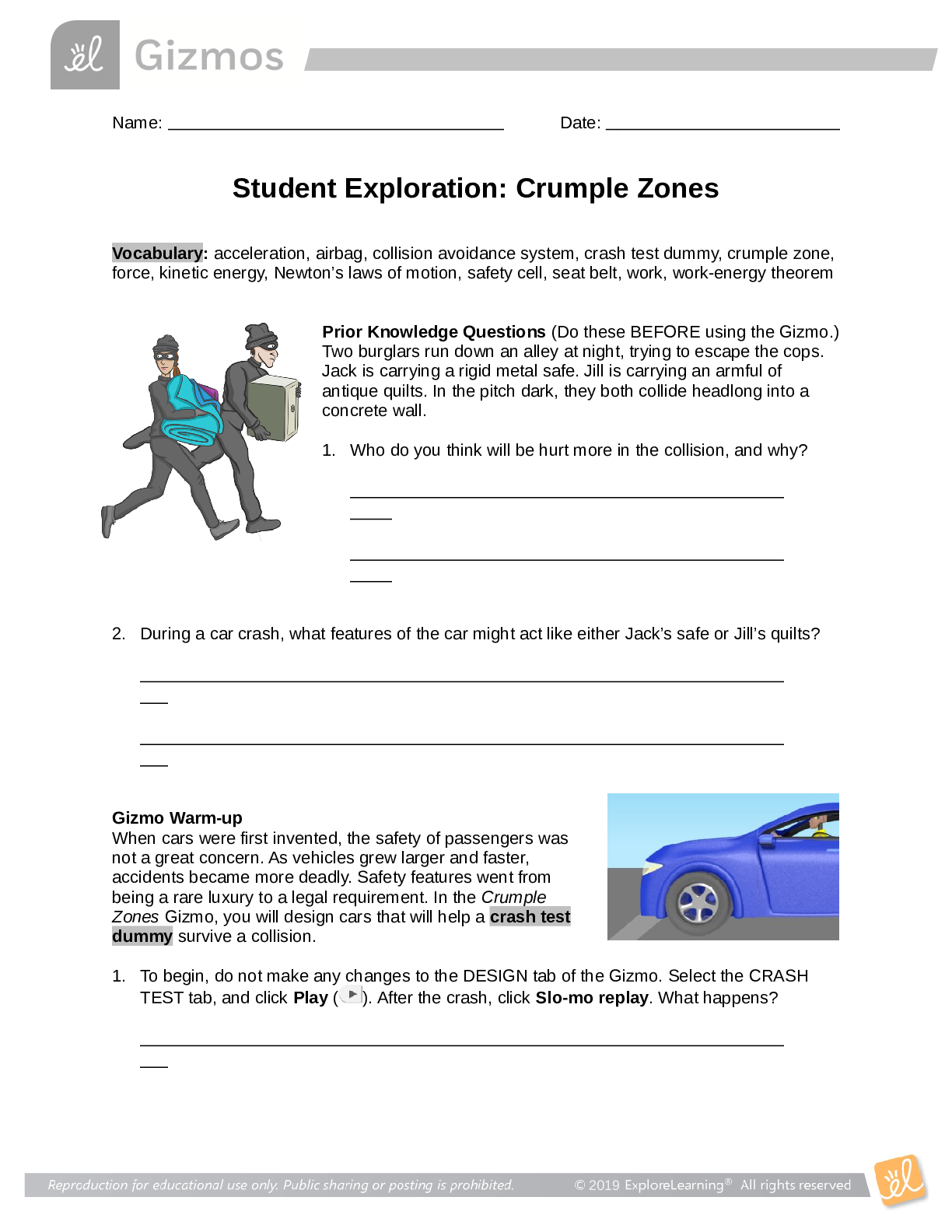
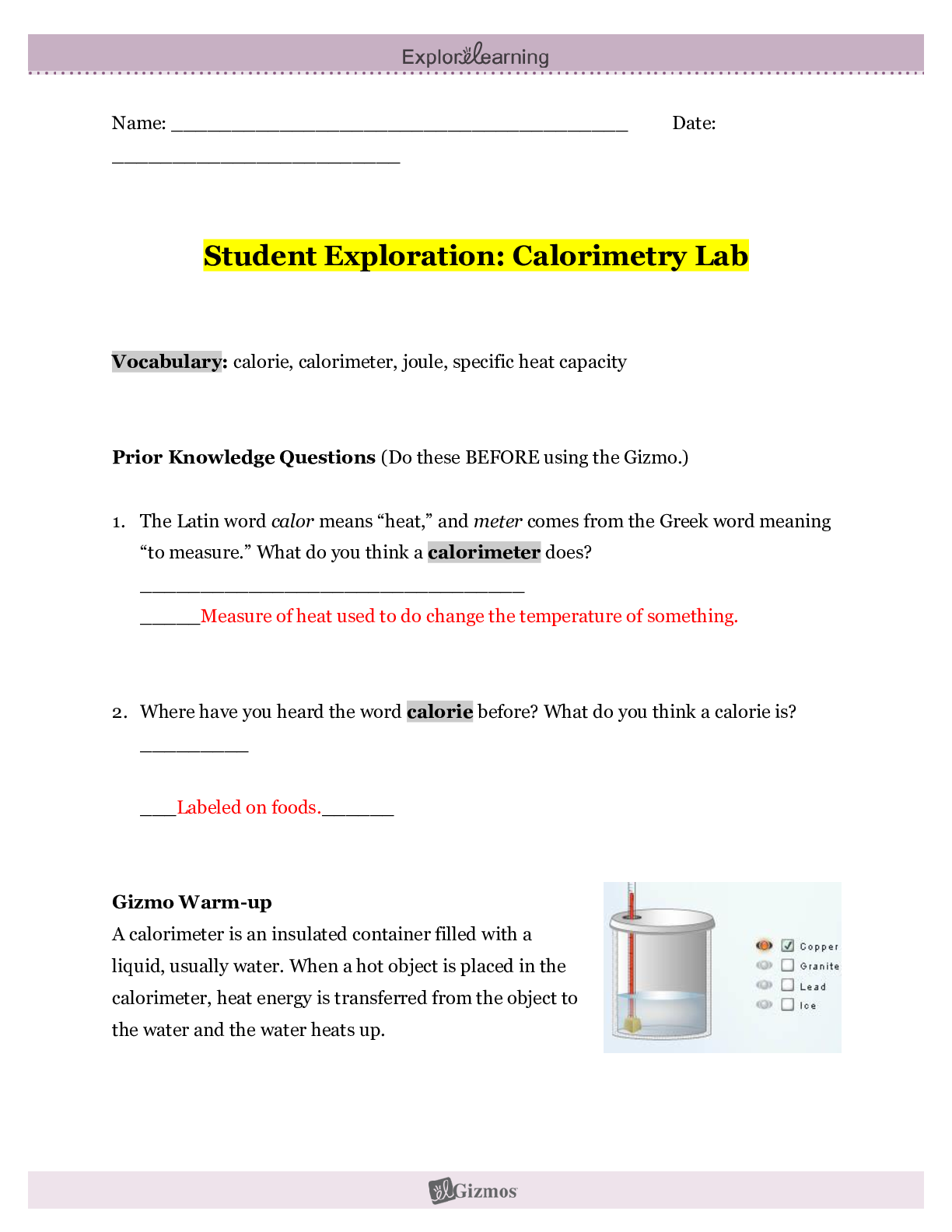
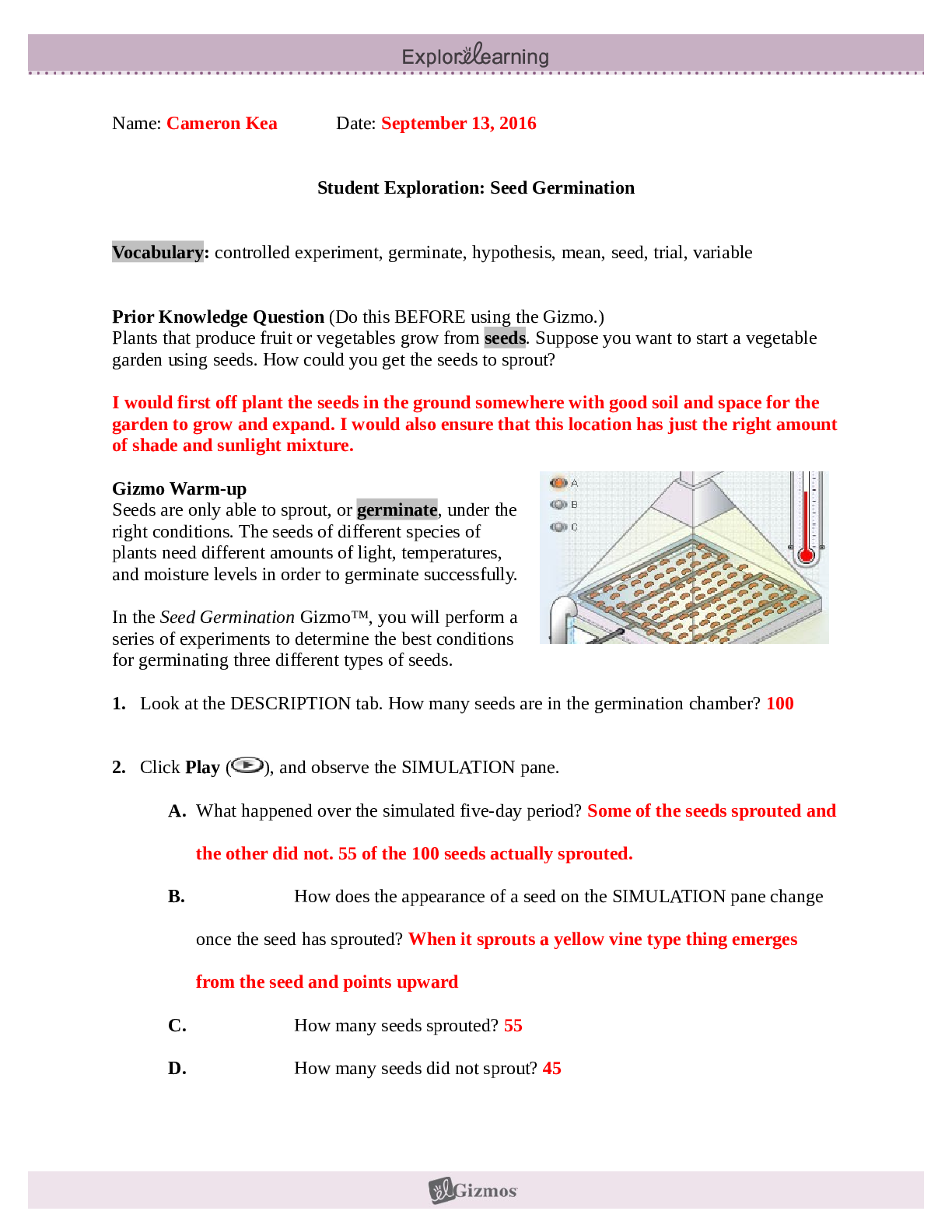
Student Exploration: Calorimetry Lab Vocabulary: calorie, calorimeter, joule, specific heat capacity Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. The Latin word calor means “he... at,” and meter comes from the Greek word meaning “to measure.” What do you think a calorimeter does? _____Measure heat___________________________ _________________________________________________________________________ 2. Where have you heard the word calorie before? What do you think a calorie is? __With food. The amount of energy it takes to heat up one ml of water. _______ _________________________________________________________________________ Gizmo Warm-up A calorimeter is an insulated container filled with a liquid, usually water. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat capacity. You will use the Calorimetry Lab Gizmo to determine the specific heat capacities of various substances. 1. On the SIMULATION pane, select Copper. Use the slider to set its Mass to 200 g. Set the Water mass to 200 g. Check that the Water temp is set to 30.0 °C and the copper’s Temp is 90 °C. Select the GRAPH tab, and click Play ( ). A. What was the Final temperature of the copper and the water? ______34.96C___________ B. How much did the temperature of the copper change? ___________55.04C_____________ C. How much did the temperature of the water change? ________4.96C_________________ 2018 2. Specific heat capacity can be described as a substance’s resistance to temperature changes. Which substance has a greater specific heat capacity, copper or water? Explain. ___Water. The temperature of the water changed the least amount, therefore its resistance is greater.______________________________________________________________________ _________________________________________________________________________ 2018 Activity A: Heat transfer Get the Gizmo ready: Click Reset ( ). Question: What factors determine how heat energy transfers between objects? 1. Predict: In the Gizmo warm-up, you saw how 200 g of 90 °C copper transfers heat to 200 g of 30.0 °C water. A. How do you think increasing the water’s mass would affect the final temperature? _________It would decrease the final temperature. __________________________________________________________ ___________________________________________________________________ B. How do you think decreasing the copper’s mass would affect the final temperature? ___________It would decrease the final temperature. ________________________________________________________ ___________________________________________________________________ C. How do you think increasing or decreasing the copper’s initial temperature would affect the final temperature? ___Increasing it would increase the final temperature, decreasing it would decrease the final temperature. ________________________ [Show More]
Last updated: 1 year ago
Preview 1 out of 13 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 10, 2022
Number of pages
13
Written in
Additional information
This document has been written for:
Uploaded
Apr 10, 2022
Downloads
0
Views
79







.png)