Chemistry > QUESTIONS & ANSWERS > North Carolina State University CH CH101 CHEMISTRY Chapter 3. Current Score : 9.67 / 10 (All)
North Carolina State University CH CH101 CHEMISTRY Chapter 3. Current Score : 9.67 / 10
Document Content and Description Below
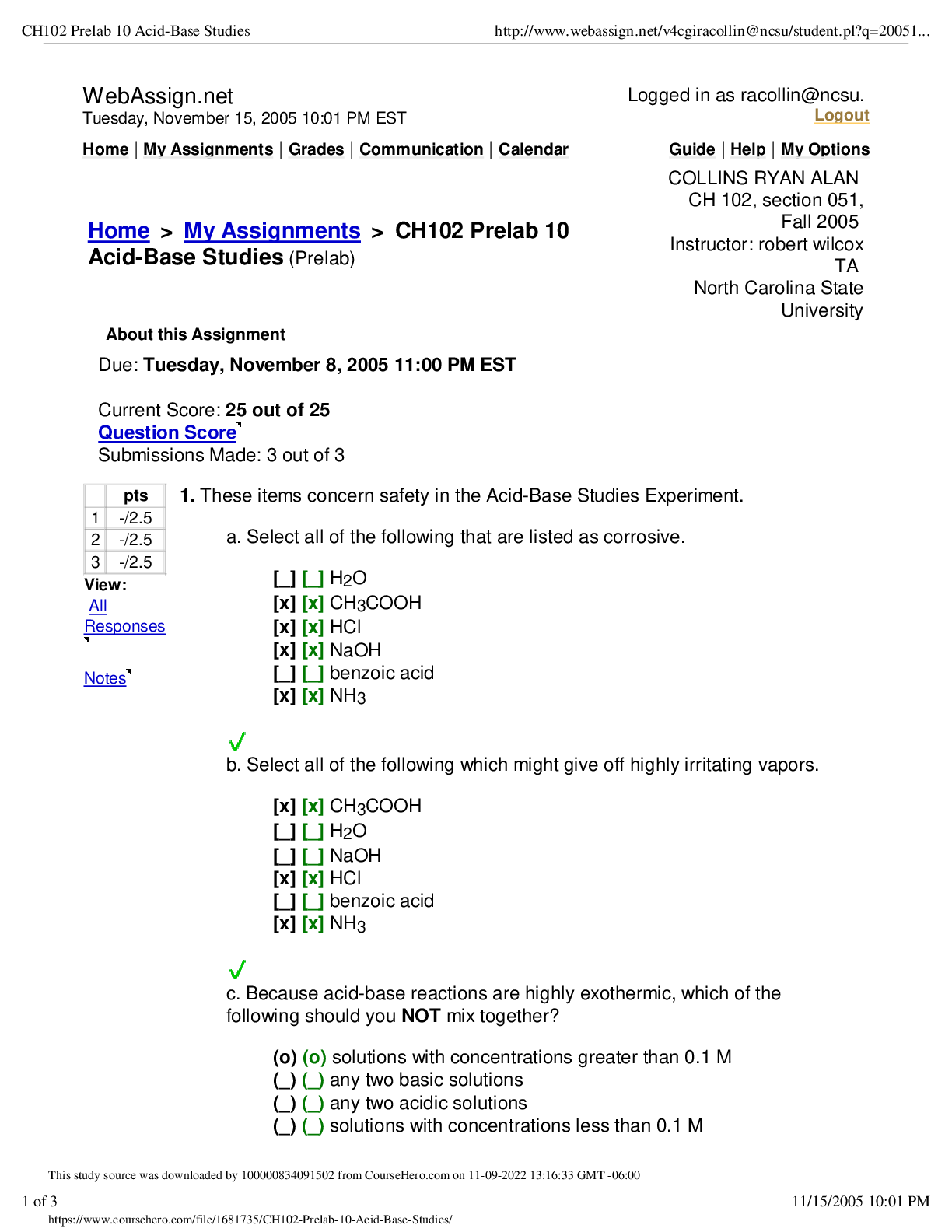
Chapter 3 http://www.webassign.net/web/Student/Assignment-Responses/view_key?dep=15304244 1/6 Current Score : 9.67 / 10 Due : Tuesday, January 31 2017 11:59 PM EST 1. 0.66/0.66 points | Previous An... swers Identify the elements (by typing in the symbol) with the following electron configurations: a) [Kr]5s24d6 Ru Ru b) [Ne]3s23p4 S S c) [Xe]6s24f145d7 Ir Ir d) [Kr]5s24d105p3 Sb Sb 2. 0.33/0.66 points | Previous Answers Base your answers to this question on the energy diagram for atoms G, H, and K. (a) Rank the atoms in ionization energy, from smallest to largest. Your response should be a three letter "word" made up of the letters G, H, and K. For example, if you think G is the smallest, followed by H, and K (the largest), your answer should be GHK (no spaces, no commas). ghk GHK (b) Rank the atoms in electron affinity, from smallest to largest. Your response should be a three letter "word" made up of the letters G, H, and K. For example, if you think G is the smallest, followed by H, and K (the largest), your answer should be GHK (no spaces, no commas) hgk KGH Chapter 3 (Homework) Forrest Singletary CH 101, section 005, Spring 2017 Instructor: Elena Jakubikova WebAssign The due date for this assignment is past. Your work can be viewed below, but no changes can be made. http://www.webassign.net/web/Student/Assignment-Responses/view_key?dep=15304244 2/6 3. 0.66/0.66 points | Previous Answers Based solely on their location on the periodic table and the predicted trends, rank the following atoms in ionization energy, from smallest to largest. Your response should be a four letter "word" made up of the letters a, b, c and d. For example, if you think a is the smallest, followed by b, c and d (the largest), your answer should be abcd (lower case, no spaces, no commas). a) Sr b) Rb c) Te d) S bacd bacd 4. 0.66/0.66 points | Previous Answers How many unpaired electrons are in a: a) zinc atom 0 0 b) nitrogen atom 3 3 c) chlorine atom 1 1 d) nickel atom 2 2 5. 0.66/0.66 points | Previous Answers Based solely on their location on the periodic table and the predicted trends, rank the following atoms in size, from smallest to largest. Your response should be a four letter "word" made up of the letters a, b, c and d. For example, if you think a is the smallest, followed by b, c and d (the largest), your answer should be abcd (lower case, no spaces, no commas). a) Na b) Si c) Rb d) S dbac dbac http://www.webassign.net/web/Student/Assignment-Responses/view_key?dep=15304244 3/6 6. 0.66/0.66 points | Previous Answers Check all of the following statements that are true. You should check anywhere from one to three boxes. 7. 0.66/0.66 points | Previous Answers Refer to the orbital energy diagrams below to answer this question. a b c Assume the orbitals below those shown are completely full, and the orbitals above those shown are completely empty. (Each of the four parts of this question are worth equal amounts.) a) Which of the diagrams represent excited state configurations? (Check all boxes that apply; there may be one, two or three correct answers. You must have this part completely correct to receive credit.) b) Write the chemical symbol of element represented by each diagram. (Note: if the diagram is for an excited state, you may want to redraw the ground state to help you answer these questions.) diagram (a) O O diagram (b) Ne Ne diagram (c) Mn Mn none of these statements is correct the 3s orbital on Na is at lower energy than the 3p orbital on Cl the 5p orbital on I is at lower energy than the 4p orbital on Br the 4s orbital on K is at lower energy than the 3s orbital on Na diagram (b) diagram (a) diagram (c) none of the diagrams represent excited states http://www.webassign.net/web/Student/Assignment-Responses/view_key?dep=15304244 4/6 8. 0.66/0.66 points | Previous Answers Check all of the following statements that are true. You should check anywhere from one to three boxes. 9. 0.66/0.66 points | Previous Answers How many total p electrons does an atom of Sn have? 20 20 How many of these p electrons are in the outermost level? [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 06, 2022
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Oct 06, 2022
Downloads
0
Views
44









.png)



.png)
.png)



.png)

.png)




.png)
.png)


