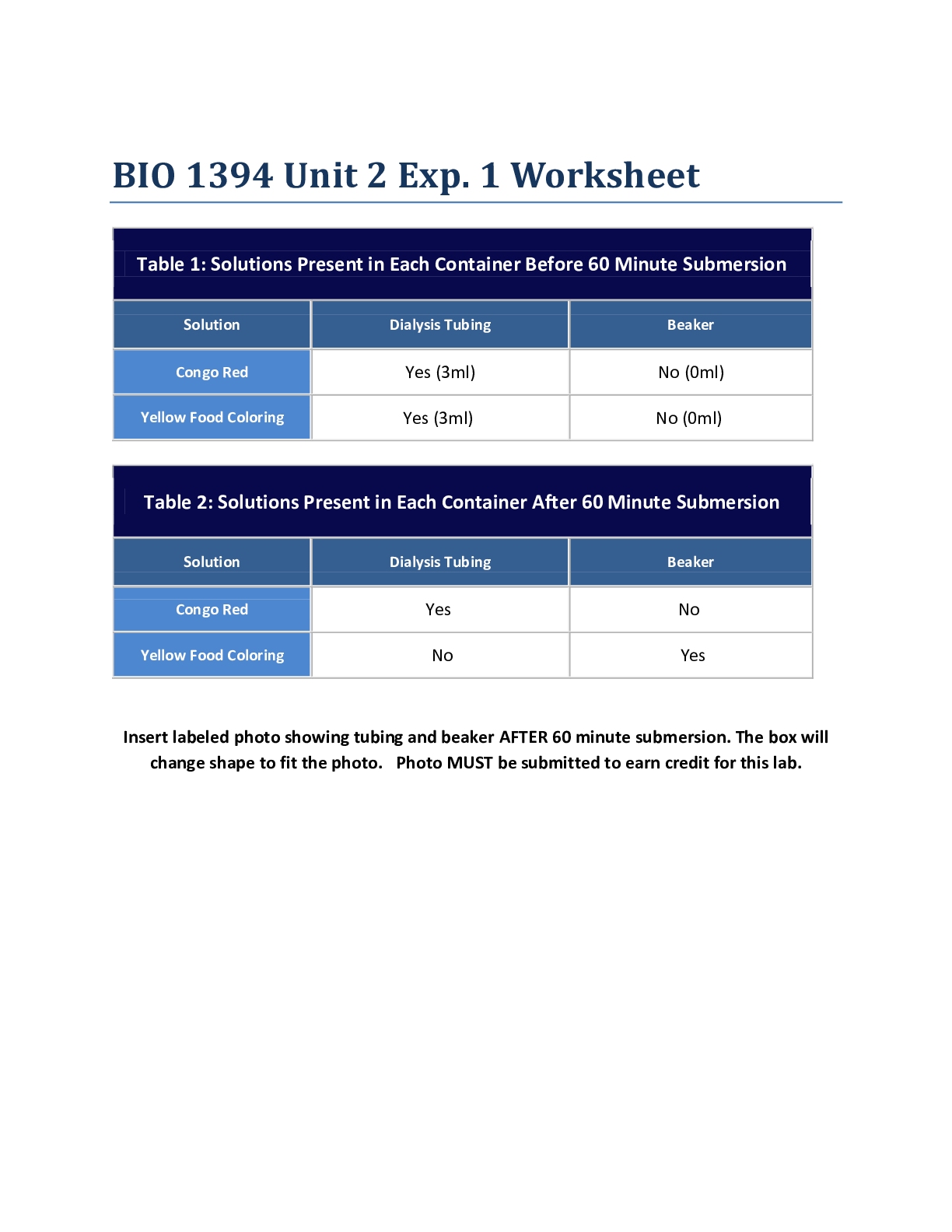
Chemistry > Lab Experiment > Northern Virginia Community College CHM 111. Calorimetry Lab Sp16. To perform simple calorimetry exp (All)
Northern Virginia Community College CHM 111. Calorimetry Lab Sp16. To perform simple calorimetry experiments. To use calorimetry results to calculate the specific heat of an unknown metal. To determine the enthalpy of neutralization for a strong acid-strong base reaction
Document Content and Description Below
Calorimetry Objectives To perform simple calorimetry experiments. To use calorimetry results to calculate the specific heat of an unknown metal. To determine the enthalpy of neutralization for a s... trong acid-strong base reaction Introduction Any chemical or physical change involves a transfer of heat (energy), where heat may exit the system (exothermic) or be absorbed by the system (endothermic). The amount of heat that flows into or out of the system is determined with a technique called calorimetry (heat measurement). A calorimeter is a laboratory apparatus that is composed of an insulated container, a thermometer, a mass of water, and the system to be studied, and is used to measure the quantity and direction of heat flow accompanying the chemical or physical change. Heat is measured in the energy units, Joules (J), defined as 1 kg.m2/s2. Another common unit is the calorie (cal) which is defined as the heat required to raise the temperature of 1-g of water by 1C. Enthalpy (or heat) of reaction, H, is the quantitative expression used to express the heat change in chemical reactions that are at constant pressure. H values are negative for exothermic reactions and positive for endothermic reactions and are often expressed as J/mol or kJ/mol. The specific heat of any substance can be determined in a calorimeter. The specific heat is an intensive physical property of a substance (independent of sample size) and is the quantity of heat necessary to raise the temperature of 1-g substance by 1C. Specific heat of some common substances are listed in Table 1. Note that either C or K can be used for the change in temperature, since the difference in a degree is the same for both scales. [Show More]
Last updated: 1 year ago
Preview 1 out of 11 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 24, 2022
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Mar 24, 2022
Downloads
0
Views
51

-2.png)
-2.png)








.png)













.png)