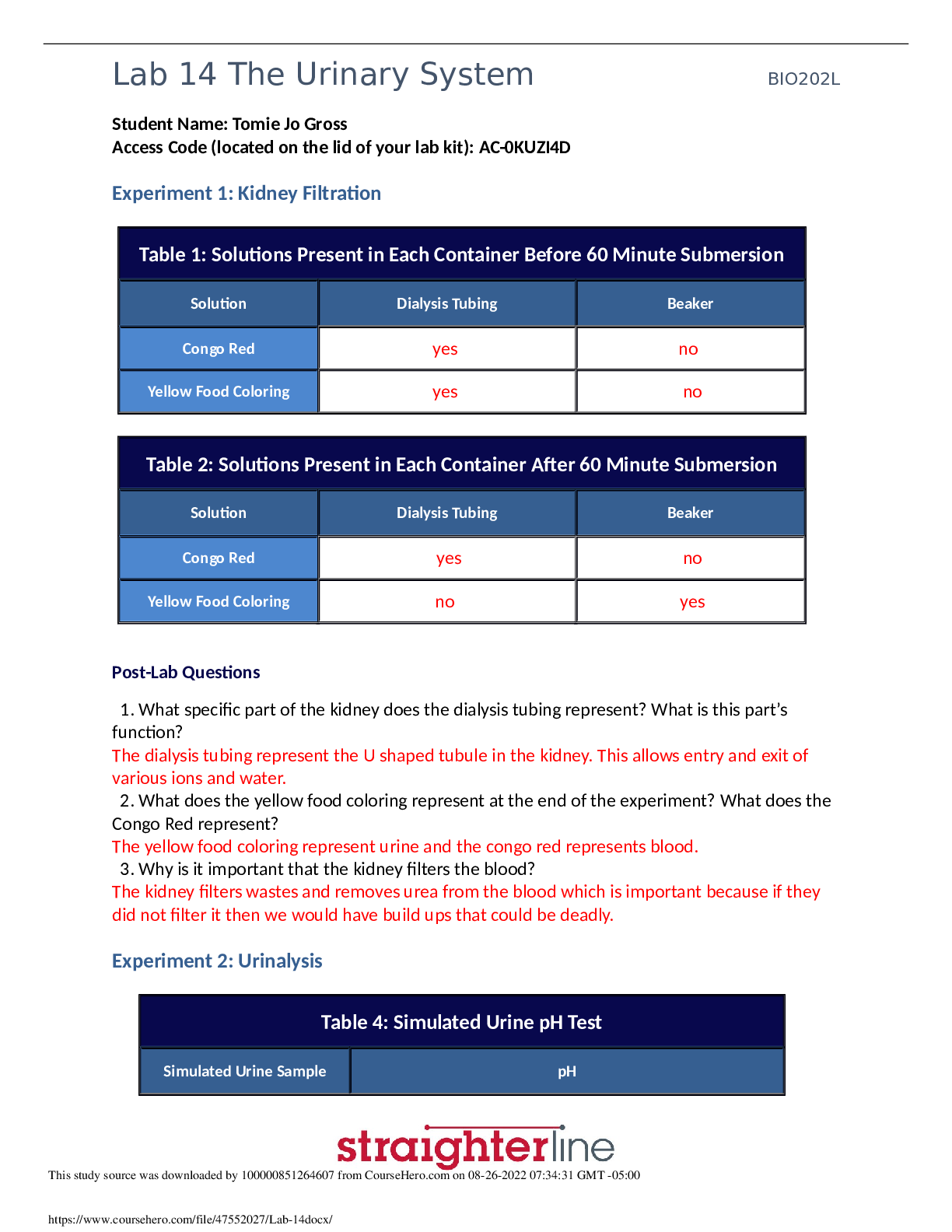
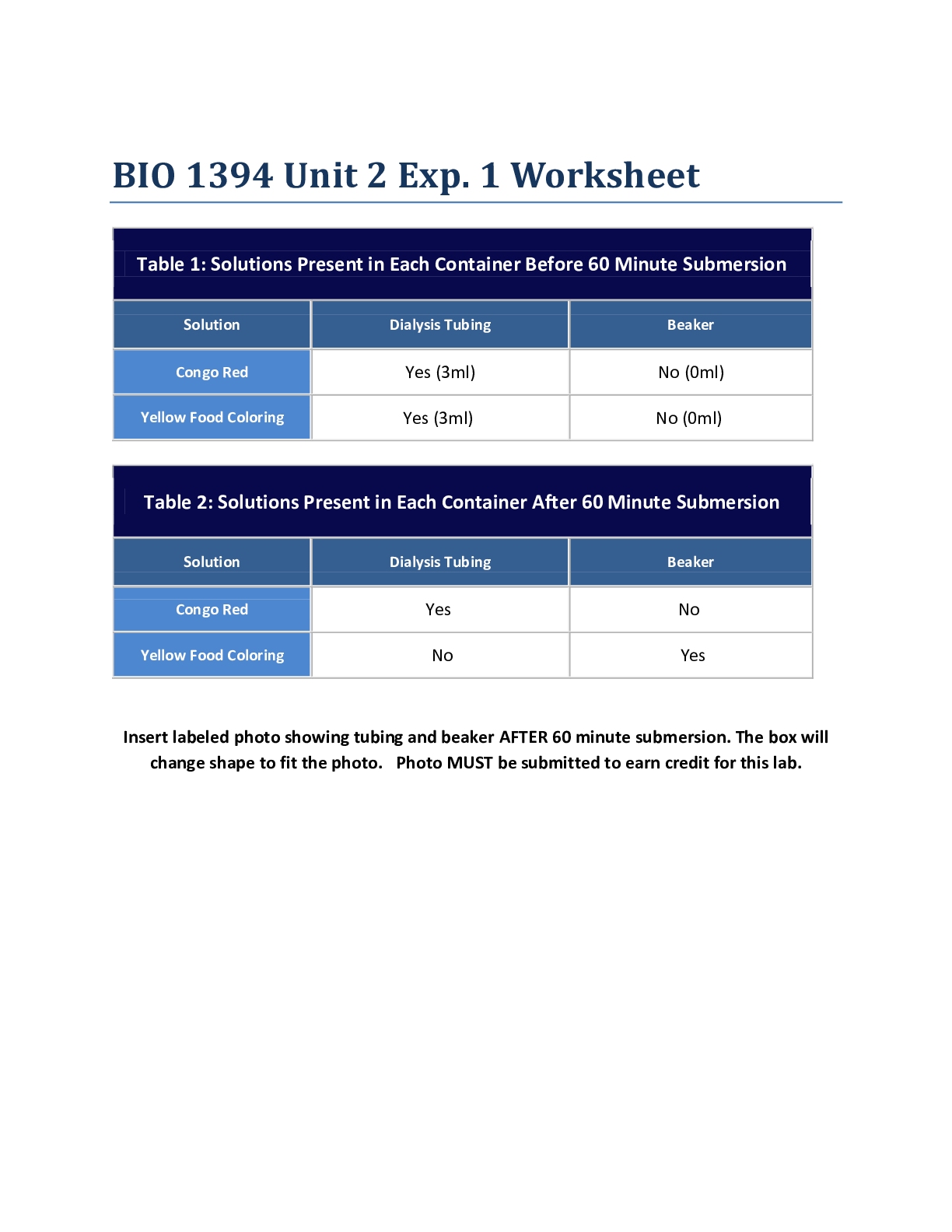
Chemistry > Lab Experiment > Lab Report > Lab 9_The Hand Warmer Design Challenge: Where Does the Heat Come From? Use chemistry to (All)
Lab Report > Lab 9_The Hand Warmer Design Challenge: Where Does the Heat Come From? Use chemistry to design an effective, safe, environmentally benign, and inexpensive hand warmer. The ideal hand warmer increases in temperature by 20°C (but no more) as quickly as possible, has a volume of about 50 mL, costs as little as possible to make, and uses chemicals that are as safe and environmentally friendly as possible. Carry out an experiment to determine which substances, in what amounts, to use in order to make a hand warmer that meets these criteria.
Document Content and Description Below
Use chemistry to design an effective, safe, environmentally benign, and inexpensive hand warmer. The ideal hand warmer increases in temperature by 20°C (but no more) as quickly as possible, has a v... olume of about 50 mL, costs as little as possible to make, and uses chemicals that are as safe and environmentally friendly as possible. Carry out an experiment to determine which substances, in what amounts, to use in order to make a hand warmer that meets these criteria. Context for This Investigation Have your fingers ever been so cold they felt numb? Wouldn’t it be great if you could generate heat to warm your hands up anytime you want to? That’s exactly what a “hand warmer” does. Hand warmers are small packets that people put inside gloves or mittens on cold days to keep their fingers warm. They are very popular with people who work outside in winter or engage in winter sports. One type of hand warmer contains water in one section of the packet and a soluble substance in another section. When the packet is squeezed the water and the soluble substance are mixed, the solid dissolves and the packet becomes warm. In this experiment, students will learn how a hand warmer works and use chemistry to design an effective, safe, environmentally benign, and inexpensive hand warmer. Pre-lab Preparation An animation showing the dissolution of an ionic compound on the particulate level can be found on the website Chemistry Experiment Simulations and Conceptual Computer Animations: https://chemdemos.uoregon.edu/demos/Calorimetry-Heat-of-Solution-Computer-Simulation After viewing the animation, answer the following questions with your group: 1. Describe the changes you observe in the animation, including changes in the bonds and particulate attractions and changes in the amount of disorder in the system. 2. When sodium chloride is dissolved in water, the temperature of the resulting solution is lower than the temperature of the water before the salt dissolves. How can this result be explained based on the bond breaking and bond making that is occurring? 3. Why do some salts, such as sodium chloride, dissolve spontaneously even though the process is endothermic overall? 4. When some ionic salts are dissolved in water, the temperature of the resulting solution is higher than the temperature of the water before the salt dissolves. What do you think determines whether the resulting solution is cooler or warmer than the starting water? [Show More]
Last updated: 1 year ago
Preview 1 out of 3 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jan 23, 2023
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Jan 23, 2023
Downloads
0
Views
135

.png)
-2.png)
-2.png)







.png)













.png)