Physics > Lab Experiment > Lab Experiment > New York University_SCIENCE: FLAME TEST AND ATOMIC SPECTRA LAB C12-2-02 (All)
Lab Experiment > New York University_SCIENCE: FLAME TEST AND ATOMIC SPECTRA LAB C12-2-02
Document Content and Description Below
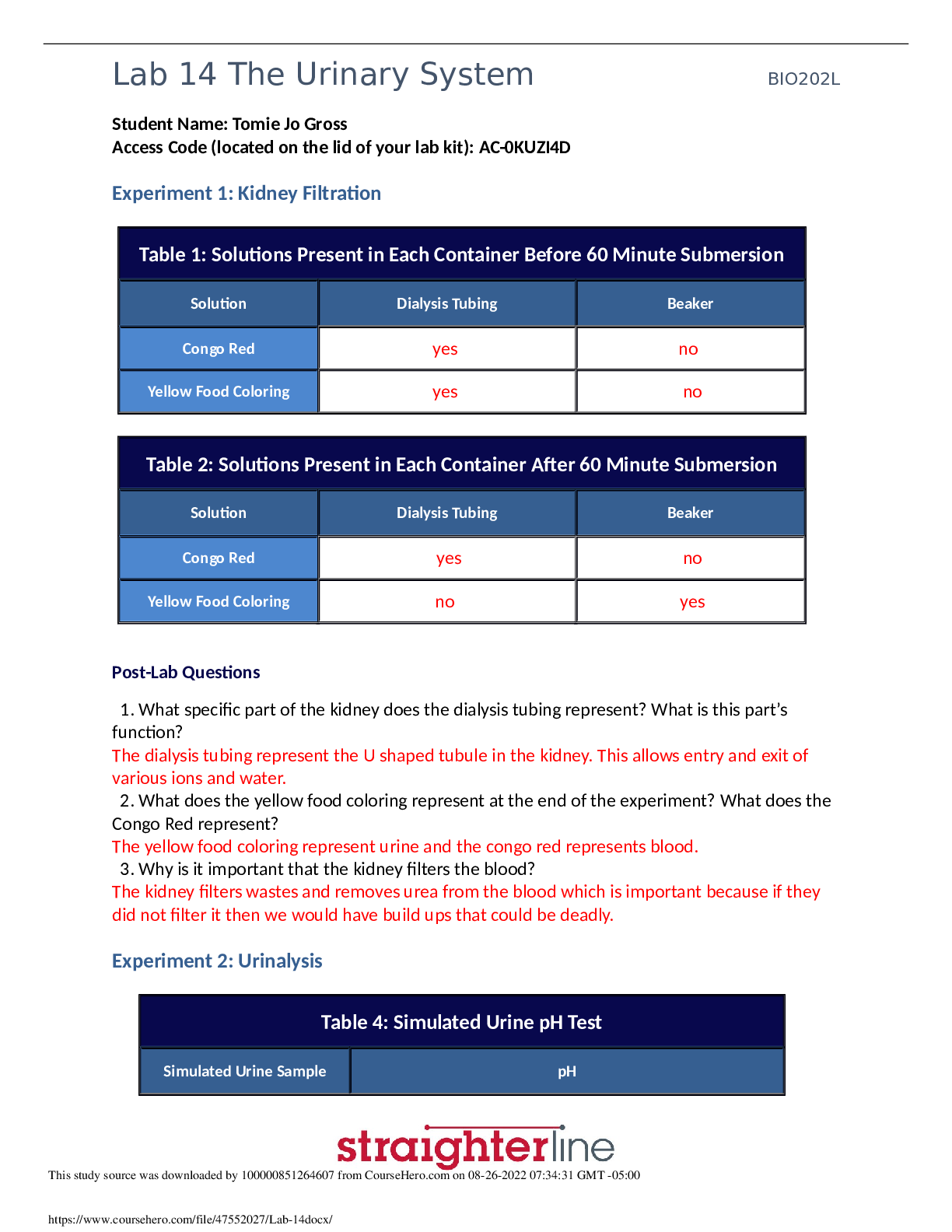
(See teacher background information in Flame Tests, Atomic Spectra and Applications Activity) Introduction: Have you ever seen a fireworks display? Where do all of the colors come from?... Below are some links to the chemistry of fireworks: http://chemistry.about.com/od/fireworkspyrotechnics/Fireworks_Pyrotechnics.htm http://alchemy.chem.uwm.edu/amalgamator/NCW/ncw2001/fireworks.html In this activity, you will investigate the colors of flame produced by solutions of metal salts. A flame test is a procedure used to test qualitatively for the presence of certain metals in chemical compounds. When the compound to be studied is excited by heating it in a flame, the metal ions will begin to emit light. Based on the emission spectrum of the element, the compound will turn the flame a characteristic color. This technique of using certain chemical compounds to color flames is widely used in pyrotechnics to produce the range of colors seen in a firework display. Certain metal ions will turn the flame very distinctive colors; these colors in turn can help identify the presence of a particular metal in a compound. However, some colors are produced by several different metals, making it hard to determine the exact ion or concentration of the ion in the compound. Some colors are very weak and are easily overpowered by stronger colors. For instance, the presence of a potassium ion in a compound will color a flame violet. But on the other hand, even trace amounts of sodium ions in a compound produce a very strong yellow flame, often times making the potassium ion very difficult to detect. To counteract the effects of any sodium impurities, one can view the flame through a piece of cobalt blue glass. The cobalt glass absorbs the yellow light given off by sodium while letting most other wavelengths of light pass through. In this activity, wooden splints dipped in solutions of metal salts are heated using a Bunsen burner, producing different colored flames. By comparing the color given off by an unknown with the known metal salts, the identity of the metal salt can be determined. Flame Tests Activity (As an option, this could be a demo rather than a student activity) Materials: Bunsen burner Wooden splints (9 per group) Solutions (1.0 mol/L) of the following metal salts Procedure: 1. Obtain a cobalt blue glass and 9 wooden splints that have been soaking in the metal salt solutions. (Why is soaking the splints important?). Be sure to label each wooden splint with the names of the salts so they are not mixed up. 2. Light the Bunsen burner and open the air vent to obtain a non-luminous flame with two blue cones. Be sure to avoid a yellow flame. (Why?) 3. Carefully place the end of the wooden splint that was soaked in the metal salt solution at the top of the inner blue cone. Record the color and intensity (bright/faint) of the flame in the data table. The color given off by the salt is the initial color observed, not the yellow-orange color produced by the burning wood. (To avoid burning the wood, wave the splint through the flame rather than holding it right in the flame). 4. Repeat with the other 8 salts. Be sure to record the colors as precisely as possible. 5. For the sodium potassium mixture, observe the colors as before and then again by looking through the cobalt glass. The cobalt glass cuts out any yellow-orange color. 6. If more observations are needed, dip the clean end of the wooden splint in the solutions for a few minutes and repeat. Otherwise, discard the wooden splints at the end of the experiment. Data Table: Analysis: The electromagnetic spectrum is shown below. Recall that energy is proportional to frequency, while frequency is inversely proportional to wavelength. Use this information to answer questions 1-4 below. List the colors observed in this lab from the highest energy to the lowest energy. List the colors observed in this lab from the highest frequency to the lowest frequency. List the colors observed in this lab from the shortest wavelength to the longest wavelength. What is the relationship between energy, frequency, and wavelength? Based on the results of your experiment, what metal was found in the unknown? Explain. What is the purpose of the cobalt blue glass? Why is only the purple color of the potassium seen through the cobalt glass? Do you think we can use the flame test to determine the identity of unknowns in a mixture? Why or why not? How are electrons “excited” in this part of the experiment? What does it mean when the electrons are “excited”? Explain why we did not see distinct lines (like on an emission spectrum) when the metal salts were burned. What particles are found in the chemicals that may be responsible for the production of colored light? Why do different chemicals emit different colors of light? Why do you think the chemicals have to be heated in the flame first before the colored light is emitted? Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions? Are these light emission applications related? Explain. Optional: Research some information about the origin of fireworks. (The websites listed at the beginning of this activity might be helpful). Explain how they are made, what chemicals are used, what color they burn and their uses. [Show More]
Last updated: 1 year ago
Preview 1 out of 4 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jan 08, 2023
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Jan 08, 2023
Downloads
0
Views
47

.png)
-2.png)
-2.png)



















.png)