Chemistry > Lab Experiment > AP Chemistry Lab Investigation #12: _Determining the Enthalpy of Chemical Substances Dissolved in Wa (All)
AP Chemistry Lab Investigation #12: _Determining the Enthalpy of Chemical Substances Dissolved in Water. The purpose of this lab is to learn how to determine the enthalpy change of different chemical substances dissolved in water stirred by the magnetic stirrer and ultimately to find the most appropriate chemical substance for hand warmer
Document Content and Description Below
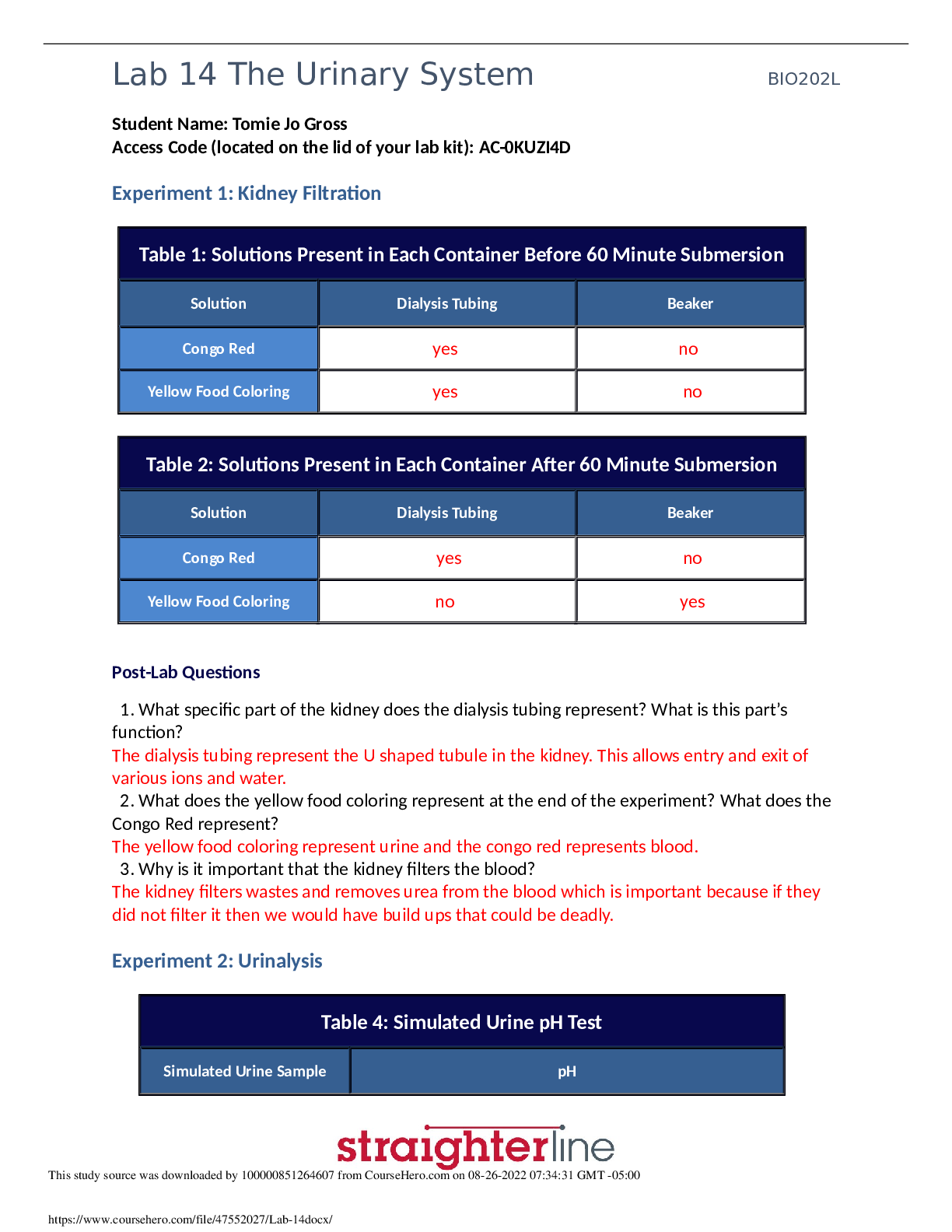
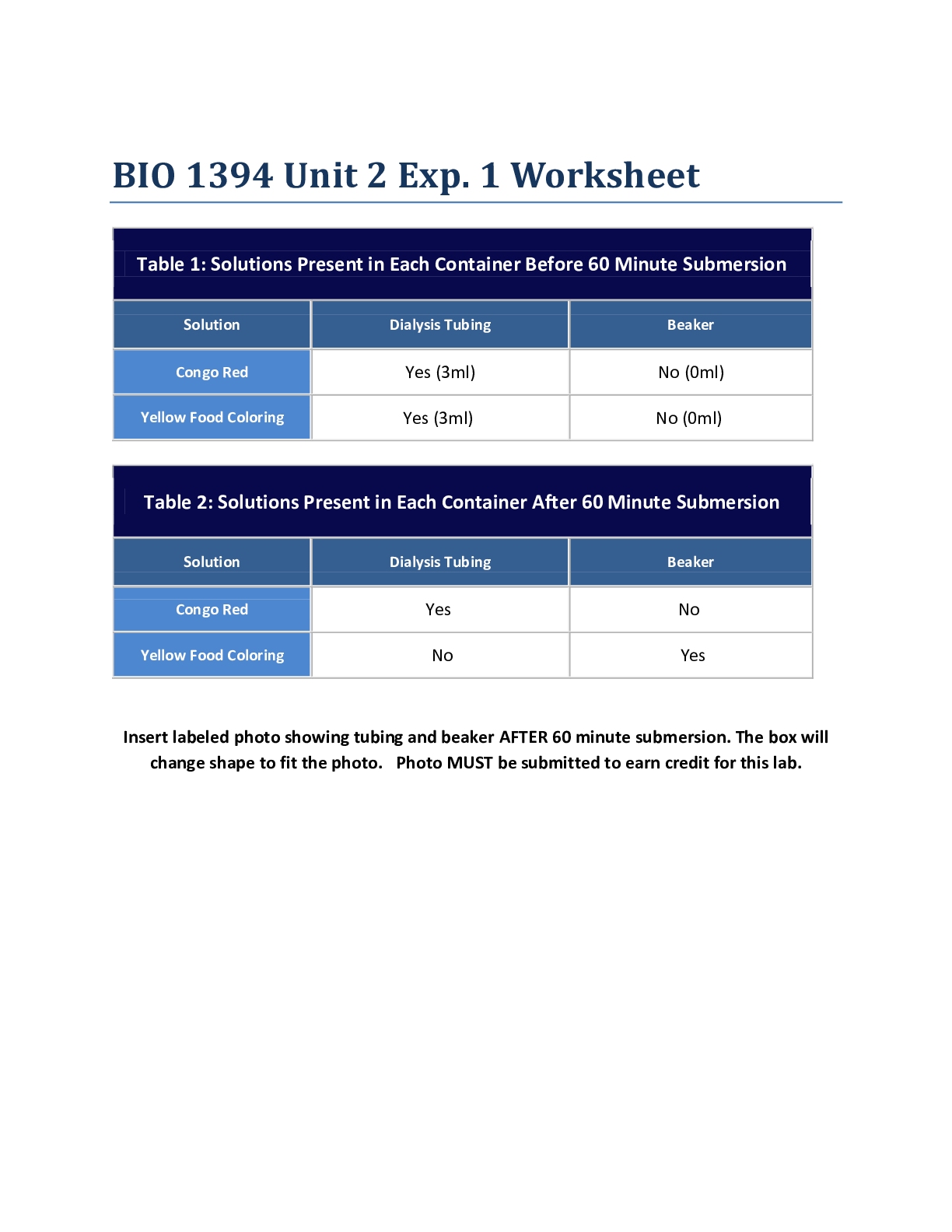
Determining the Enthalpy of Chemical Substances Dissolved in Water Abstract: The purpose of this lab is to learn how to determine the enthalpy change of different chemical substances dissolved in w... ater stirred by the magnetic stirrer and ultimately to find the most appropriate chemical substance for hand warmer. This was accomplished by measuring the temperature change of dilutions, substituting the given data (mass, ΔT, specific heat capacity) into the mathematical equation: q=mcΔT. The enthalpy change for each chemical was then calculated by comparing the flow of heat (q) for each chemical. AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee TABLE OF CONTENTS Materials 2 Procedure 3 Pre-Lab Questions 3 Data Collection and Computation 5 Argumentation and Documentation 7 Post-Lab Questions 8 1AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee MATERIALS: - Thermometer - Balance - Scoops - 100mL graduated cylinder - 150mL beaker - 150mL Styrofoam calorimeter with Styrofoam cover (see Figure 1) - Weighing boat - Magnetic stirrer (see Figure 1) and stir bar - Anhydrous calcium chloride (CaCl2)* - Anhydrous sodium carbonate (N2CO3) - Anhydrous sodium acetate (NaC2H3O2) - Lithium chloride (LiCl)* - Magnesium sulfate (MgSO4)* - Sodium chloride (NaCl) (*Among the seven chemicals, we’ve used three of them for the experiment, which are boldly highlighted: anhydrous calcium chloride, lithium chloride, and magnesium sulfate.) Figure 1: Styrofoam calorimeter on the magnetic stirrer. PROCEDURES: 2AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee - Part 1 (calorimetry practice): We made a series of solutions by putting 5 grams of each chemical substance (in our case: MgSO4, LiCl, and CaCl2) into 100mL of distilled water (about 24-25 ℃) within a Styrofoam calorimeter. The Styrofoam calorimeter contained one magnetic bar inside and it sat on the magnetic stirrer (see Figure 1). Then, we turned the magnetic stirrer on, covered the lid, and fixed the thermometer with the container, observing the temperature change until the temperature stopped changing. We repeated this experiment twice for each chemical substance. Finally, given the temperature change, we calculated the flow of heat (q=mc ΔT) and then calculated the enthalpy change per mole (ΔH kJ/mol) by dividing the calculated value of q by molar mass of each chemical substance. Part 2 (calorimeter calibration procedure): We first measured 50mL of distilled water at room temperature (about 24-25℃) in a graduated cylinder. Then, we prepared for hot water that was approximately 60-70℃ and poured into the same graduated cylinder that contained cold water little by little. We did this until the temperature reached 50℃. When the desired temperature was approached, we measured the mixed water to be 100mL and then poured it into the Styrofoam calorimeter, observing the temperature change until it stopped changing. Given the temperature change, we calculated the q of cold water and hot water so that we could calculate the q of calorimeter by using the equation: qhot=-(qcold+qcal). PRE-LAB QUESTIONS: 1. Describe the changes you observe in the animation, including changes in the bonds and particulate attractions and changes in the amount of disorder in the system. 2. When sodium chloride is dissolved in water, the temperature of the resulting solution is lower than the temperature of the water before the salt dissolves. How can this result be explained based on bond breaking and bond making that is occurring? 3AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 3. Why do some salts, such as sodium chloride, dissolve spontaneously even though the process is endothermic overall? 4. When some ionic salts are dissolved in water the temperature of the resulting solution is higher than the temperature of the water before the salt dissolves. What do you think determines whether the resulting solution is cooler or warmer than the starting water? a) Calculate the enthalpy change of the cold water using the equation qhot=mhotc∆thot. Assume that the density of water is exactly 1g/mL. Is this an endothermic or exothermic process? Explain. b) Calculate the enthalpy change of the hot water using the equation qcold=mcoldc∆tcold. Assume that the density of water is exactly 1g/mL. Is this an endothermic or exothermic process? Explain. c) These amounts are not equal because the calorimeter (the coffee cups) absorbs some of the thermal energy transferred by the hot water. Thus under the real conditions observed in the laboratory the law of conservation of energy equation becomes qhot= -(qcold+qcal), where qcal is the enthalpy change of the calorimeter. Use this equation to calculate the enthalpy change of the calorimeter. d) The calorimeter constant, C, is the heat absorbed by the calorimeter per degree of temperature change, C=qcal/∆tcal. Assuming the starting of the temperature of the calorimeter is the same as that of the cold water, calculate the calorimeter constant in units of joules per degree Celsius. 5 AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee 3. By convention, scientists report enthalpy changes for dissolution (and many other processes) in units of kilojoules per mole of solute dissolved. Using your values of qsoln, calculate the enthalpy in units of kilojoules per mole. This quantity has the symbol ∆Hsoln. Calculate ∆Hsoln for each of the three solids you tested. 4. Based on the cost information provided, and your experimental work and calculations, select which chemical you believe will make the most cost-effective hand warmer. The hand warmer you are designing needs to increase in temperature by 20°C. Calculate the amount of the compound you selected that would be required for a hand warmer that meets this requirement. 6 AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee ARGUMENTATION AND DOCUMENTATION: Out of all the substances that we have tested in the experiment, we came up with a conclusion that CaCl2 is the most appropriate chemical that qualifies all aspects -safety, cost, and heatto make a hand warmer. This is because CaCl2 released the most amount of energy, at same conditions, compared with other substances. In other words, it had the highest Enthalpy (kJ/mol) of an average of -38.49; while other chemicals such as LiCl had -33.32 and also MgSO4 with -31.73. CaCl2 is not only suitable to make an effective hand warmer for its amount of heat released per mole, but also for its cheap price. CaCl2 costs $6.55 per 500 grams which is relatively cheaper than other substances like LiCl –the second highest enthalpy- which costs $32.75 per 500 grams (see Table 2). According to the Material Safety Data Sheets (MSDS), CaCl2 is non-flammable, but it has potential effects to harm human health in case of skin contact, eye contact, ingestion, and inhalation in extreme rare cases. Therefore, the precautions might be to keep locked up, do not ingest, breathe dust, avoid contact with eye and skin, and keep away from incompatibles such as moisture. Overall, the data values of enthalpies were all measured based on the experimented results, and the safety and environmental impact were based on MSDS. All dilutions, including those of other classmates, were made with same volume of Styrofoam calorimeters. The class-average value of heat enthalpy of CaCl2 turns out to be about -40.23 kJ/mol, which is not numerically distant from our data: -38.49 kJ/mol. However, this value seems far from the published value, which is -81.3 kJ/mol, a percentage error of about 50.5% error. The equipment and methods utilized for this experiment were generally same for whole students. Therefore, neither the amounts of CaCl2 nor the indicated heat enthalpy of CaCl2 was not appropriate for this experiment. This would not, however, change our conclusion since we know that the actual heat enthalpy for CaCl2 is over -80 kJ/mol, which is found to be more effective than we expected. POST-LAB ASSESSMENT: 7 AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee 1. Are the dissolving processes you carried out endothermic or exothermic or neither? Explain your thinking. 2. Dissolving ionic compounds involves the separation of the solid ionic compound into cations and anions in water. This process can be represented by an equation showing the solid as a reactant and the aqueous ions as products. The heat of reaction ∆Hsoln is written after the products, typically in units of kJ/mol. Example: sodium hydroxide dissolves exothermically, releasing 44.2 kilojoules per mole dissolved. This process is represented as NaOH(s) + Na+ (aq) + OH- (aq), ∆Hsoln =-44.2 kJ/mol. Write an equation to represent the dissolving process for each salt you studied. Include your calculated heat of reaction 3. Changes in matter are generally classified as physical or chemical based on whether new substances are formed through the process. Does dissolving represent a physical change, a chemical change, or an intermediate change? Explain your reasoning, including evidence from the animation you viewed. 4. Share your calculated values of ∆Hsoln with your classmates and obtain their values. a) Determine the class average value and standard deviation* for each solid. 8 AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee b) Find the published value of ∆Hsoln for each solid and determine the percent error in the class average. 9 AP Chemistry Lab Investigation #12: Heat enthalpy 2017-09-29 By Aiden Moon, Gloria Yoo, James Lee 5. What possible sources of error could affect the accuracy of your calculated value of the amount of solid in your hand warmer? List at least two and what effect they would have on the temperature change. [Show More]
Last updated: 1 year ago
Preview 1 out of 11 pages
-2.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jan 23, 2023
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Jan 23, 2023
Downloads
0
Views
105

.png)
-2.png)








.png)












.png)