Chemistry > QUESTIONS & ANSWERS > CHEM 120 Week 1_ 7 Quizzes (Version 1) Quiz Bank : Latest 2020/21Complete solutions. (All)
CHEM 120 Week 1_ 7 Quizzes (Version 1) Quiz Bank : Latest 2020/21Complete solutions.
Document Content and Description Below
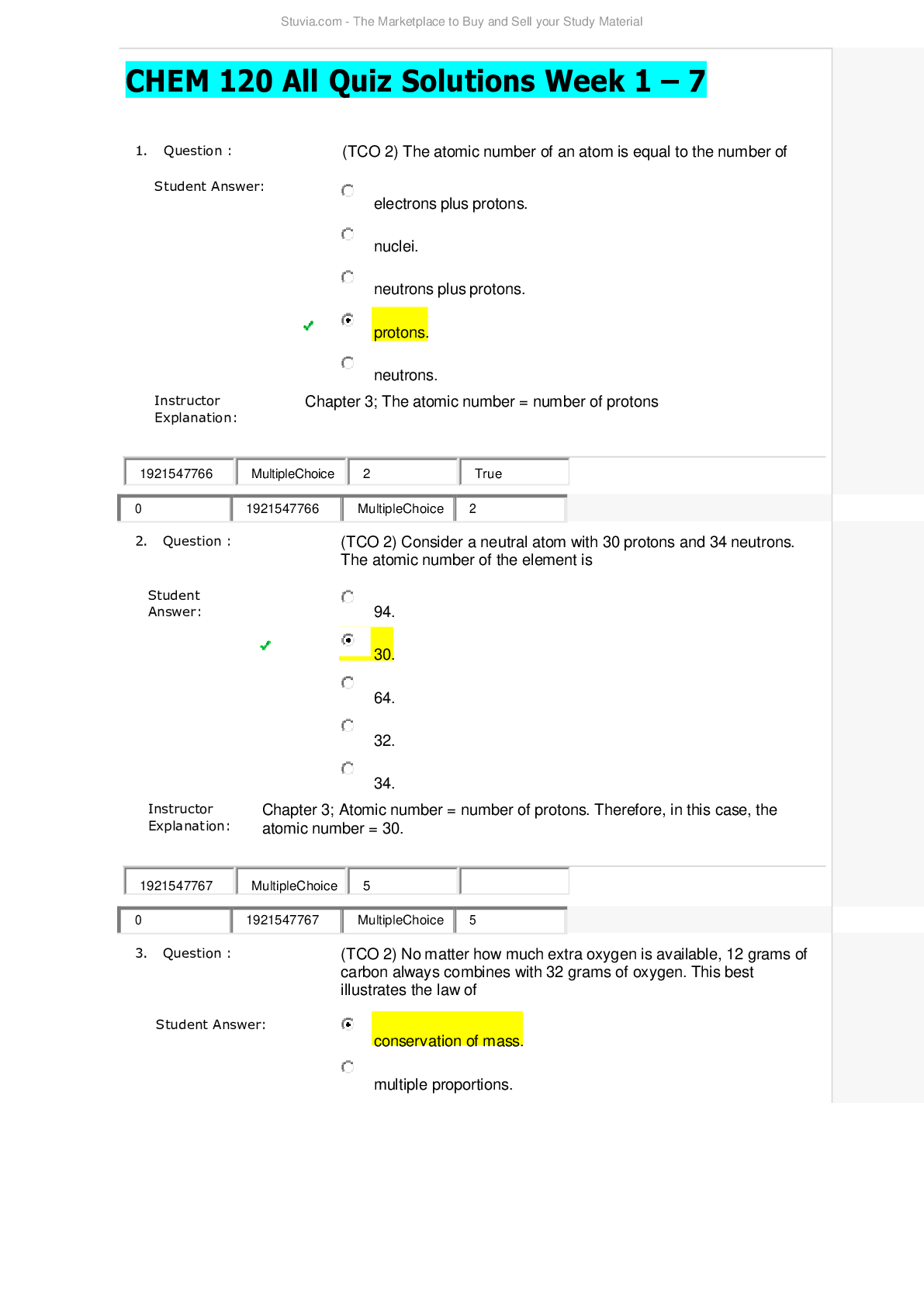
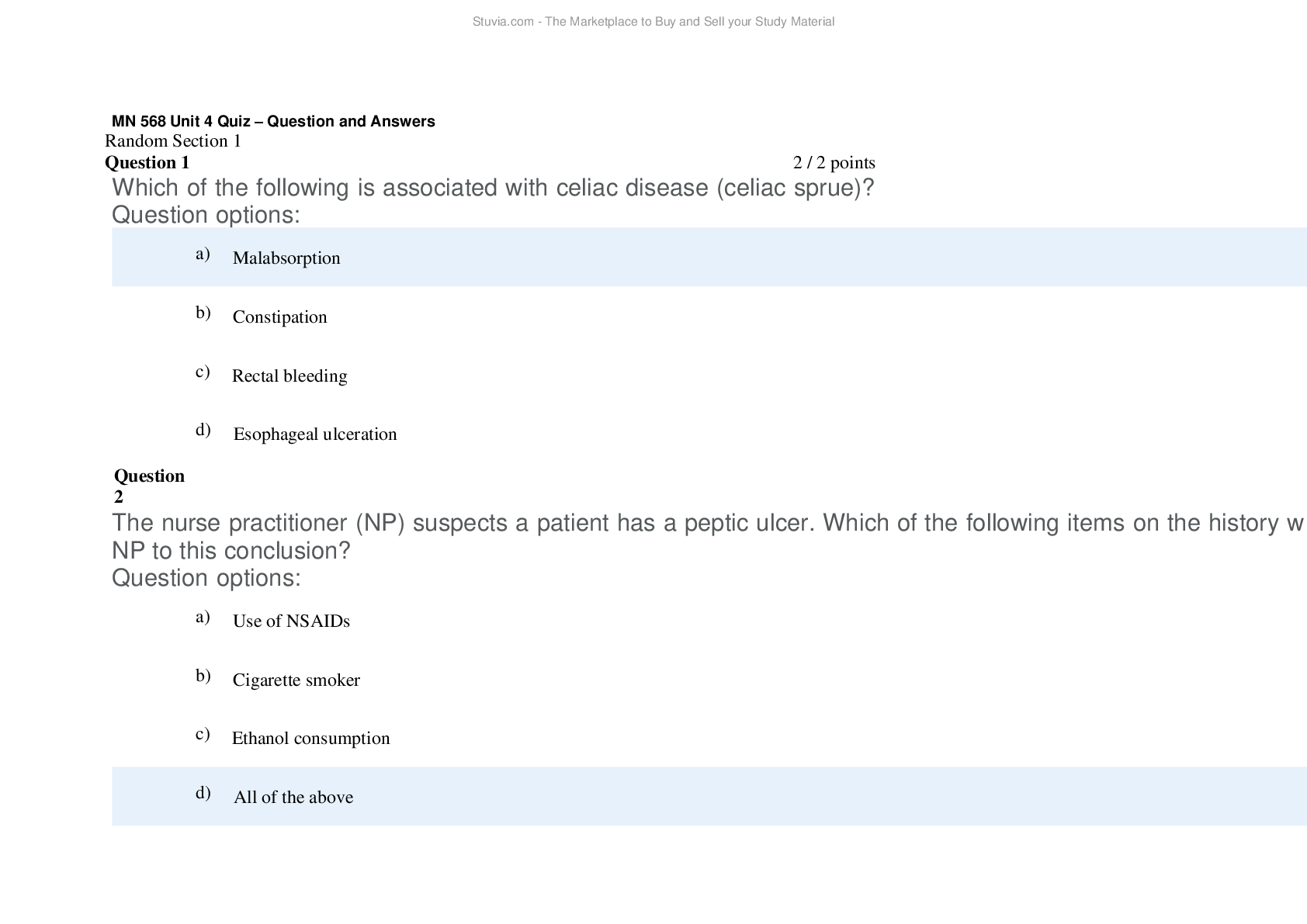
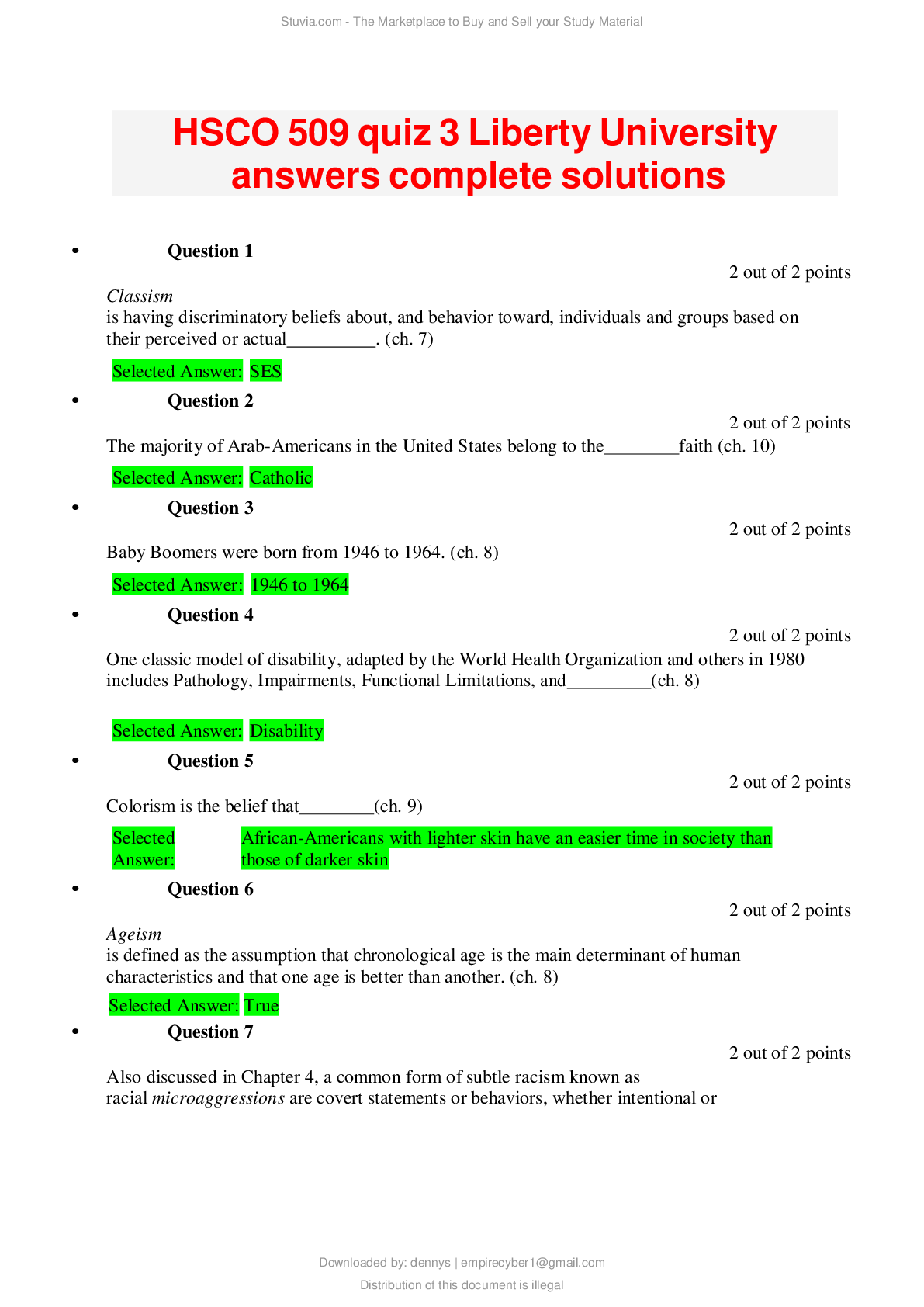
CHEM 120 Weekly Quizzes (Version 1) 1. Question : (TCO 1) You have been growing the same variety of corn for over 5 years. In the past two years, you have noticed that the yield has decreased by 10% e... ach year. You grow corn on 10 acres of land, and your yield should be 1,000 bushels per acre. Growing conditions have been consistent for the past 5 years…… hypothesize that CAQ will produce 1,000 bushels per acre. Design an experiment to test this hypothesis. Include your control(s) (2 points) and your experimental design (4 points). Describe the experimental results that would support your hypothesis (2 points). Be specific. 2. Question : (TCO 2) Determine the number of protons, neutrons, and electron for the element shown below. The mass number for the isotope of this element is 90. You must show your work for each determination to receive credit (2 points per determination). 3. Question : (TCO 1) What is the mass of 74 mL of ethyl alcohol, which has a density of 0.79 g/mL? Show your work. 4. Question : (TCO 1) What is the mass of 53 mL of ethyl alcohol, which has a density of 0.79 g 5. Question : (TCO 1) How many quarts are in 12.5 liters? Show your work. 6. Question : (TCO 1) How many meters are in 7 yards? Show your work. 7. Question : (TCO 2) The atomic number of an element is 8. Question : (TCO 2) Isotopes are atoms of the same element with 9. Question : (TCO 2) The proton has 10. Question : (TCO 2) Which type of radioactivity has a negative charge? 11. Question : (TCO 2) No matter how much extra oxygen is available, 12 grams of carbon always combines with 32 grams of oxygen. This best illustrates the law of 12. Question : (TCO 2) Consider a neutral atom with 30 protons and 34 neutrons. The atomic number of the element is 13. Question : (TCO 2) The atomic number of an atom is equal to the number of 14. Question : (TCO 3) The maximum number of electrons that may reside in the n=2 energy level is 15. Question : (TCO 3) How many electrons are in a phosphorus atom? 16. Question : (TCO 3) Which is an impossible electron configuration? 17. Question : (TCO 3) The ground element with the ground state electron configuration of 1s2 2s2 2p6 3s2 is 18. Question : (TCO 3) Which substance has ionic bonds? 19. Question : (TCO 3) A oxide ion is _____. 20. Question : (TCO 3) The correct name of the compound AlCl3 is _____. 21. Question : (TCO 4) If a central atom has a total of two groups and no lone pairs attached to it, the geometry about the central atom is _____. 22. Question : (TCO 4) Which of the following is the electron dot structure for nitrogen, N2? 23. Question : (TCO 4) Which substance has nonpolar covalent bonds? 24. Question : (TCO 3) What is the name of the following compound: LiBr? 25. Question : (TCO 3) What is the name of the following compound: PCl5? 26. Question : (TCO 3) What is the name of the following compound: BaSO4? 27. Question : (TCO 3) Write the chemical formula for the following compound: sodium carbonate. NOTE: You will not be able to use subscripts to note the number of atoms. This is OK. 1. Question : (TCO 5) A reaction that releases energy as it occurs is classified as a(n) _____. 2. Question : (TCO 5) What should be the coefficient of hydrogen, H2, in the following equation in order to make it balanced? 2 Al 3 H2SO4 —> Al2(SO4)3 ? H2 3. Question : (TCO 5) Magnesium reacts with oxygen to form magnesium oxide and has the following balanced chemical equation: 2 Mg O2 –> 2 MgO. How many mole(s) of MgO are produced when 0.20 mole O2 reacts completely? 4. Question : (TCO 5) Determine the # of atoms for each element present in the following molecule: Cu(NO2)2 (3 pts.). Using the periodic table of elements, determine the molar mass for this molecule (7 pts.). 5. Question : (TCO 7) You are asked to calculate the concentration of a KCl (in water) solution in several ways and are given the following information: 3.5 g KCl, 50 mL solution, 50 g water, 1 mole KCl = 74.6 g KCl. (1) Calculate the mass/mass percent concentration (4 pts.). (2) Calculate the Molarity of the solution (6 pts.). 6. Question : (TCO 7) What volume (L) of a 3M KOH solution can be prepared by diluting 0.5 L of a 5M KOH solution? 1. Question : (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the acid? CO32- H2O -> OH- HCO3- 2. Question : (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the base? H3PO4 NH3 -> NH4 H2PO4- 3. Question : (TCO 8) What is reduced in the following reaction? 2 Bi3 3 Mg -> 2 Bi 3 Mg2 4. Question : (TCO 8) What is the pH of a solution with a [H3O ] of 1 x 10-9 M? 5. Question : (TCO 6) A sample of helium gas occupies 1245 mL at 705 mmHg. For a gas sample at constant temperature, determine the volume of helium at 745 mmHg. 6. Question : (TCO 6) A gas at a temperature of 85 degrees C occupies a volume of 175 mL. Assuming constant pressure, determine the volume at 15 degrees C. 7. Question : (TCO 6) For a gas at standard temperature and pressure with a density of 2.75 g/L, determine its molar mass. 8. Question : (TCO 6) Calculate the pressure, in atmospheres, of 3.94 mol CO(g) in a 4.25 L tank at 31 degrees C. 1. Question : (TCO 9) What type of compound is CH3-CH2-CH3? 2. Question : (TCO 9) What is the name of CH3-CH2-CH2-CH3? 3. Question : (TCO 9) Name the following organic compound: CH3CH2CH2CH2CH2CH3. 4. Question : (TCO 9) Name the following organic compound: CH3CH2NH2. 5. Question : (TCO 10) Match the organic compound with its use or characteristic. 6. Question : (TCO 9) Match the organic compound with the functional group found in that molecule. 1. Question : (TCO 12) Celluloid is a chemical modification of which natural product? 2. Question : (TCO 12) High-density polyethylene is composed of 3. Question : (TCO 12) Natural rubber can be made from the monomer 4. Question : (TCO 12) Which of the following is NOT true of composite materials? 5. Question : (TCO 12) The monomer of polypropylene is 6. Question : (TCO 11) The process of positron emission can be considered as the 7. Question : (TCO 11) Nitrogen-13 has a half-life of 10 minutes. How much of a 16 mg sample would remain after 30 minutes? 8. Question : (TCO 11) Sodium-24 has a half-life of 15 hours. How much of a 40 mg sample would remain after three half-lives? 9. Question : (TCO 11) Which of the following does NOT result in an increase in entropy? 10. Question : (TCO 11) The fact that a refrigerator requires energy to move heat from a colder object (inside of the refrigerator) to a hotter object (outside of the refrigerator) is a real life observation of which thermodynamic law? 11. Question : (TCO 11) Radon (Rn) has a mass number of 222 and an atomic number of 86 and decays by emission of an alpha particle. The product of this decay is 12. Question : (TCO 12) Compare and contrast the molecular structure and performance characteristics of LDPE and HDPE. Provide an example of each type of polymer (2 pts). 13. Question : (TCO 12) Describe two types of methods that can be used to mold plastic products. 14. Question : (TCO 11) Thorium (Th), with a mass number of 224 and an atomic number of 90, decays by emission of an alpha particle. Identify the product of the nuclear reaction by providing its atomic symbol (2 pts), mass number (2 pts), and atomic number (1 pt). 1. Question : (TCO 9) Which of the following compounds is an alkyne? 2. Question : (TCO 9) What is the name of this compound? CH3-CH2-CH2-CH2-CH3? 3. Question : (TCO 9) Name the following organic compound: CH3CH2CH2CH2CH2CH3. 4. Question : (TCO 9) Name the following organic compound: CH3CH2CH2CH2CH2COOH. 5. Question : (TCO 10) Match the organic compound with its use or characteristic. 6. Question : (TCO 9) Match the organic compound with the functional group found in that molecule. 1. Question : (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the acid? CO32- H2O -> OH- HCO3- 2. Question : (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the base? H3PO4 H2O -> H3O H2PO4- 3. Question : (TCO 8) What is reduced in the following reaction? Cl2 2 NaBr -> 2 NaCl Br2 4. Question : (TCO 8) What is the pH of a solution with a [H3O ] of 1 x 10-12 M? 5. Question : (TCO 6) A sample of helium gas occupies 1521 mL at 719 mmHg. For a gas sample at constant temperature, determine the volume of helium at 745 mmHg. 6. Question : (TCO 6) A gas at a temperature of 85 degrees C occupies a volume of 175 mL. Assuming constant pressure, determine the volume at 15 degrees C. 7. Question : (TCO 6) For a gas at standard temperature and pressure with a density of 4.55 g/L, determine its molar mass. 8. Question : (TCO 6) Calculate the volume, in liters, of a 2.12 mol H2S(g) at 60 degrees C and 1.38 atm. 1. Question : (TCO 5) Which of the following factors will increase the rate of a reaction? 2. Question : (TCO 5) What should be the coefficient of hydrogen, H2, in the following equation in order to make it balanced? 2 Al 3 H2SO4 —> Al2(SO4)3 ? H2 3. Question : (TCO 5) Magnesium reacts with oxygen to form magnesium oxide and has the following balanced chemical equation: 2 Mg O2 –> 2 MgO. How many mole(s) of oxygen gas (O2) are needed to react with 4.0 moles of Mg? 4. Question : (TCO 5) Determine the # of atoms for each element present in the following molecule: Al(NO3)2 (3 pts.). Using the periodic table of elements, determine the molar mass for this molecule (7 pts.). 5. Question : (TCO 7) You are asked to calculate the concentration of a KCl (in water) solution in several ways and are given the following information: 3.5 g KCl, 50 mL solution, 50 g water, 1 mole KCl = 74.6 g KCl. (1) Calculate the mass/mass percent concentration (4 pts.). (2) Calculate the Molarity of the solution (6 pts.). 6. Question : (TCO 7) What volume (mL) of a 3.5% (m/v) KOH solution can be prepared by diluting 45.0 mL of a 15% (m/v) KOH solution? 1. Question : (TCO 3) The maximum number of electrons that may reside in the n=1 energy level is 2. Question : (TCO 3) How many electrons are in a potassium atom? 3. Question : (TCO 3) Which is an impossible electron configuration? 4. Question : (TCO 3) The ground element with the ground state electron configuration of 1s2 2s2 2p6 3s2 is 5. Question : (TCO 3) Which substance has ionic bonds? 6. Question : (TCO 3) A nitride ion is _____. 7. Question : (TCO 3) The correct name for the compound N2O3 is _____. 8. Question : (TCO 4) Which of the following is the electron dot structure for hydrogen, H2? 9. Question : (TCO 4) Which substance has polar covalent bonds? 10. Question : (TCO 3) What is the name of the following compound: MgO? 11. Question : (TCO 3) What is the name of the following compound: SiBr4? 12. Question : (TCO 3) What is the name of the following compound: BaSO4? 13. Question : (TCO 3) Write the chemical formula for the following compound: lithium phosphate. NOTE: You will not be able to use subscripts to note the number of atoms. This is OK. 1. Question : (TCO 2) The mass number of an atom can be calculated from 2. Question : (TCO 2) Consider a neutral atom with 30 protons and 34 neutrons. The mass number for this atom is 3. Question : (TCO 2) Heptane is always composed of 84% carbon and 16% hydrogen. This illustrates the law of 4. Question : (TCO 2) Which type of radioactivity has essentially no mass? 5. Question : (TCO 2) Essentially, all of the mass of an atom is due to the 6. Question : (TCO 2) Which subatomic particles have approximately the same mass? 7. Question : (TCO 2) The atomic number of an element is 8. Question : (TCO 1) How many liters are in 2.7 quarts? Show your work. 9. Question : (TCO 1) How many grams are in 35 ounces? Show your work. 10. Question : (TCO 1) Diamond has a density of 3.52 g/mL. What is the volume in mL of a diamond with a mass of 15.1 g? Show your work. 11. Question : (TCO 1) What is the mass of 74 mL of ethyl alcohol, which has a density of 0.79 g/mL? Show your work. 12. Question : (TCO 2) Determine the number of protons, neutrons, and electron for the element shown below. The mass number for the isotope of this element is 81. You must show your work for each determination to receive credit (2 points per determination). 13. Question : (TCO 1) You have been growing the same variety of corn for over 5 years. In the past two years, you have noticed that the yield has decreased by 10% each year ..........................continued,,,,download to boost your grades [Show More]
Last updated: 1 year ago
Preview 1 out of 73 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 20, 2021
Number of pages
73
Written in
Additional information
This document has been written for:
Uploaded
May 20, 2021
Downloads
0
Views
41




















.png)

.png)




.png)
.png)

.png)

